What place is there for the history of science in a science curriculum

What place is there for the history of science in a science curriculum for citizenship?
Stein Dankert Kolstø
Department of applied education, University of Bergen
20. February 2001
Millikan’s apparatus
Introduction
In this talk I will discuss the history of science as a science curriculum component. In judging its relevance I will use its potential role for citizenship as the main criterion. The concept of citizenship needs some clarification though.
One of the main arguments for science education for all is the argument from democracy
(Driver, Leach, Millar, & Scott, 1996; Fullinwider, 1987; Millar, 1996; Sjøberg, 1997). This argument is sometimes also discussed under the heading “Science for citizenship” (Sjøberg,
1997). The democratic argument focuses on the role of science in society. One characteristic of our society is the practical, social and political issues related to environmental problems or to risks to human health. Many of these issues have a science dimension, and are often just called socio-scientific issues. Millar, Osborne and Nott (1998) states that “For most people, their contact with science is the many socio-scientific issues that confront them both as individuals and as members of society” (p. 19). It might therefore be argued that lay people would benefit from knowledge of science and the nature of science. Such knowledge will hopefully enable them to make decisions on socio-scientific issues based on an understanding and critical examination of the science-related arguments involved.
My intent in this lecture is therefor to discuss what role the history of science might play in a science curriculum aiming at empowering lay people to deal fruitfully with socioscientific issues. I will not delimit the discussion to practical and political issues, but also relate to issues of more philosophical and ethical character.
In accordance with the general phrasing of the title for this lecture, I will choose not to discuss specific teaching models, and will therefore neither relate my discussion to specific kinds of learners or age groups. I will focus more generally on potential advantages and problems related to the inclusion of the history of science in a science curriculum for citizenship. The realism of getting included the history of science in a science curriculum and in science teaching will therefore not be discussed either. Due to time constrains I will neither discuss explicitly the educational value of reproduction of historical experiments.
To give us a better idea of what narratives from the history of science might provide, I will start out my discussion by spending some time on one specific story that has fascinated me personally: the quest for the nature of electric charge. The story is better known as
Millikan’s oil drop experiment or the “Battle over the electron”. I hope this narrative will make the discussion less abstract, as I will use it to illustrate different points I want to make.
Having told my story, I will focus on some potential benefit the inclusion of the history of science in a science curriculum might provide. Thereafter I will raise a few “warning signs” that I believe is important to appoint when including the history of science in a science curriculum. Finally I will conclude the discussion by giving my personal point of view on the issue.
Before I tell about Millikan’s oil drop experiment I want to put the topic of this lecture in relief. A few years ago Eijkelhof and Gaskell (James et al., 1997), among others, performed a large study identifying “interesting and significant innovations” in science, mathematics and technology education. Significantly, none of the innovations included an emphasis of the history of science!
Now to my story!
Millikan’s oil drop experiment
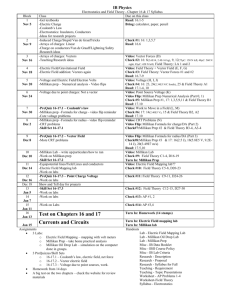
Millikan’s oil drop experiment is an interesting experiment in the history of physics, and with a fascinating story. In the beginning of the twentieth century physicists were still in doubt whether there existed of a minimum quantum of electric charge, carried by a particle called electron . The American physicist Robert Andrews Millikan decided in 1908 to start working on the problem. He sat up an experiment where he injected small charged droplets of oil into a vertical electric field between two parallel metal plates (as seen in figure 1). The idea was that the gravitational force would try to make the oil drops fall downwards, while the electric field would try to make the droplets move upwards due to their charge.
2
Figure 1. The drawing shows a sketch of the main features of Millikan’s apparatus
(Øgrim et. al. 1985).
By adjusting the strength of the electric field he could make droplets of oil rise slowly in the space between the two metal plates. He then measured the speed with which they rose and observed the voltage used. From these data he could calculate the charge on the oil drop. Each time he performed an experiment on an oil drop, he found the charge on the droplets to have the anticipated value of the charge of the electron multiplied by an integer. Through his oildrop experiment Millikan thus managed to demonstrate that there exists a minimum charge carried by the electron.
This story I have told is a rather short one. However, there are also other versions of this narrative! I will now tell on of these; one that is more detailed and contains perhaps surprising elements.
The ”Battle over the electron”
The research and disputes leading to the acceptance of the existence of a minimum charge is often called the “Battle over the electron”. In this version of the story I will draw heavily on
Gerald Holton’s (1978) account. In fact this story begins back in ancient Greece, where the natural philosopher Democritus proclaimed that matter was composed of indestructible particles. But that is a long history, and I will cut it in short and begin in 1908, when Millikan started to work on the problem.
In Europe at that time there were many physicists, among them Ernst Mach, who wanted a science free from “unnecessary” metaphysical hypotheses such as atomism (Holton, 1978).
Continuity was preferred from philosophical reasons (Bernal, 1978). Another of these
European physicists was an accomplished young man named Felix Ehrenhaft, associate professor at the University of Vienna. He was also experimenting to find out the nature of charge.
Millikan, on the other hand, was a rather unknown American physicist at the University of Chicago. He was eleven years older then Ehrenhaft, and with few scientific publications.
His philosophy was not as sophisticated as that of Ehrenhaft. Millikan confessed to a pragmatic, straightforward emphasis on “concrete visualisations” (Holton, 1978 p. 36). When
Millikan decided to work on the charge of the electron, he was well aware that the existence of the electric charge quantum was regarded as a fundamental question in physics at that time.
In his first method, which Millikan took over from Wilson, one did not look at single oil drops, but on the falling of the top layer of a whole cloud with charged water droplets. Using this method Millikan and his student Begeman found a value for the electric charge of the electron somewhat below the expected and with a high spread in the measurements. High spreads could be seen to indicate that charges of all different sizes existed, and not only integral values of a minimum quantum. He was criticised for the way he made the cloud of droplets, and started to improve the apparatus he used. Among the improvements he made was the use of higher voltage to produce a stronger electric field. But when he turned the new field on for the first time, something happened. The cloud in his apparatus just dissipated, instantaneously and completely.
At first he thought the experiment was ruined, but looking into the apparatus he observed that a few individual droplets remained in view. He suddenly realised that he had made a break through! The existence of remaining droplets could make it possible to make measurement on individual droplets with charge due to only one or a few individual electrons!
In his own words: “[seeing the droplet] moving upward with the smallest speed that it could take on, I could be certain that just one isolated electron was sitting on its back” (Millikan,
1951 p.99).
But soon he observed a new strange phenomenon. Sometimes charged water droplets floating in the electric field suddenly changed their motion radically! The discontinuity of this
3
observation was interesting. In fact it fitted well with the hypothesised discontinuity in the concept of quantified charge. Again in Millikan’s own words: “I had seen a balanced drop suddenly catch an ion [from the surrounding air.” (sited in Holton, 1978 p. 38).
The value Millikan found for the electric minimum charge was very close to the anticipated value, and he got it published in a well-known journal. In the paper Millikan also gave an account of how he judged his different measurements (se figure 2): “The observations marked with a triple star are those which were marked ’best’ in my notebook....” And also:
“Second, I have discarded three observations which I took on unbalanced drops” (sited in
Holton, 1978 p. 53) (because he judged them to be uncertain). Holton (1978) comments these judgements the following way: ”Millikan was evidently saying that he knew a good run when he saw one.” (p.53). This is an interesting point, which I will come back to later.
Figure 2. This is a picture from one of Millikan’s papers showing some of his measurements (Reproduced in Holton, 1978 p. 55)
In the paper Millikan was critical to the accuracy of results published by Ehrenhaft, even if both Ehrenhaft’s method and results at this time was rather similar to his own. (Millikan argued that Ehrenhaft’s method made it necessary to make two sets of measurements on each charged particle, with and without the electric field present, thus increasing the uncertainties.)
Millikan’s dismissal of Ehrenhaft’s work soon received an answer. In Ehrenhaft’s next article he recalculated the charge on each drop in Millikan’s paper. Instead of using the average of measured voltages as Millikan had done, he calculated the charge for each single droplet. The result was a large spread of values of the charge on the drops. This result weakened the claim for the existence of a minimum charge.
Ehrenhaft’s own experimental results, which he reported in the same paper, were remarkable. Ehrenhaft found droplets of liquid, metal particles and other small objects to have charges of half, a fifth, a tenth, a hundredth, and even a thousandth that of the electron. He concluded that indivisible quantities of electric charge did not exist at the level found by
Millikan.
But meanwhile Millikan had continued to improve his method. With this improved method Millikan, together with his assistant Fletcher, could publish new and very accurate
4
results. They reported to have “found in every case the original charge on the drop [to be] an exact multiple of the smallest charge which we found that the drop caught from the air.” (sited in Holton, 1978 p. 60). But again he frankly explains that some measurements had been excluded because they yielded values of the electric minimum quantum to low compared to their other findings.
May be surprisingly, physicists discussing Ehrenhaft’s method were not able to suggest wronging in his method. In retrospective his results nevertheless seems to have been wrong.
One important difference between their methods though, where their use of data. Holton argues that the problem seemed to be that Ehrenhaft and his colleagues used all their collected readings, good, bad, and indifferent. An example from one of Ehrenhaft’s papers is given in figure 3 (reproduced in Holton, 1978 p.74). As the curves covers all values, the figure clearly shows that a range of different charges was measured.
Figure 3. This is a picture from one of Ehrenhaft’s papers showing some of his results from measurements of electric charge (Reproduced in Holton, 1978 p. 74).
In his third major paper Millikan announced that the uncertainty in his value for the elementary charge, according to his new measurements, was 0.03 percent. This was indeed very accurate results. From his notebooks it is clear that he also this time had made judgements as to the quality of the different runs. He often wrote down evaluations like i.e. that “battery voltage dropped”; “stopwatch error occur”; and “the distance must be kept more constant” (sited in Holton, 1978 p. 69). At one occasion he wrote “ e [calculated elementary charge] = 4.98 which means that this could not have been an oil drop.” (sited in Holton, 1978 p. 69) (it could for instance have been dust in the chamber). He comments the different runs by writing i.e.: “Error high will not use.”; “Very low Something wrong”; “Exactly right”;
Publish this Beautiful one”; “Beauty. Publish this surely, beautiful!” (sited in Holton, 1978 p.
67-71).
From Ehrenhaft’s view it is Millikan’s strongly held assumption of electric charge to be
“atomised” which forces him to assume further, without proof, a high ‘error’ sometimes to be present. In Millikan’s view Ehrenhaft’s way of using data “would force one to turn one’s back on a basic fact of nature – the integral character of e [the charge of the electron]” (Holton,
1978 p. 69).
5
Millikan’s results became an important step forward to the establishing of the unitary nature of electric charge. In 1924 he received the Nobel price in physics, partly for his work on the charge of the electron.
Places for the history of science
I will now turn to explore what possibilities narratives like the “Battle over the electron” might provide for the teaching and learning of science for citizenship . Of course, the history of science contains many stories, and care should be taken not to base conclusions on one single example.
I will focus on three main arguments for the inclusion of history of science in a science curriculum for citizenship . The first relates to the nature of science, the second to the nature of interactions between science and society and technology, the third relates to the need for knowledge of our common cultural framework.
Several science educators have pointed to the inclusion of the history of science in science teaching as a motivating factor and source of variation (Holton, 1995; Millar &
Osborne, 1998; Shamos, 1995). The inclusion of human actors with hopes, struggles and success probably have a motivating effect on many learners, but this issue will nevertheless not be emphasised here.
The nature of science
My first argument is that knowledge of the nature of science is relevant for citizenship. A widespread myth about science is that scientific knowledge is true in an absolute sense
(Aikenhead, 1987; Bauer, 1994; Irwin, 2000). Scientific knowledge production is often pictured as a more or less straight foreword collection of facts followed by a logical analysis, which results in a scientific theory (Monk & Osborne, 1997). As Millar and Wynne (1988) have formulated it:
Clearly, many people believe that science is rather simple, at least in the sense that the rules are clear, and that if one follows them (which of course requires competence) the automatic result is valid, universal scientific knowledge. (p.395)
I will argue that this view of science and scientific knowledge is not well suited to understand expert disagreement, uncertainties related to scientific measurements and results, and why science often need years and decades to reach conclusions. In addition such views are not in accordance with contemporary scholarly views on the nature of science.
When introducing the nature of science as a curriculum component we have to be aware that insights brought by science studies do not represent new “facts” about science. The new insights represents hypothesis and issues to reflect upon. I will nevertheless identify a few specific claims and issues for inclusion in a science curriculum.
I believe the following interrelated learning goals related to the nature of science to be of relevance for citizens in today’s society:
1.
Scientific concepts are constructed by humans.
2.
Observations and theories are mutual dependent.
3.
Argumentation is an important part of science.
4.
Science makes use of a plurality of methods.
What might the history of science have to offer in relation to these learning goals? Concerning the first point, I believe the Battle over the electron showed that the idea that charge is atomised, that is; build up of indivisible elements, was not induced from the experimental data. On the contrary, Millikan had this idea when he started out his experiments, and the idea
6
could be traced back to ancient Greece. We also saw that other researchers held a different view. Humans as inventors of our scientific concepts are also beautifully illustrated in Galileo
Galilei’s choice of concept for acceleration (Arons, 1988). But the Millikan story might also be used to illustrate that some ideas, or hypothesis, are better then others when we are to try to describe nature.
It also seemed like Millikan had to hold his idea strongly, over a long period of time, to be able to gain convincing results. Similarly Ehrenhaft held strongly to his hypothesis, and interpreted his findings to be in accordance with his idea of continuity. Concerning the second point therefore, the Battle over the electron illustrates two important points. First, that theories guides the collection of data. Second, that the interpretation of collected data also is guided by theory. But in addition we also saw that observations, in the end, had an impact on the final acceptance of one and only one of the theories.
As to the third goal, the Millikan story at least indicates that there was a lively debate within the community of physicists both on the philosophical aspects of the different theories, and on Millikan’s and Ehrenhaft’s methodologies and experimental findings. The necessity to convince his colleagues stimulated Millikan continuously to improve his apparatus and methodology. The different results of Millikan and Ehrenhaft forced the scientific community to argue and debate the trustworthiness of the different theories, methodologies, interpretations and results. The Millikan story therefore indicates that narratives from the history of science might be used to illustrate the important role argumentation and criticism plays in scientific knowledge production.
Concerning the fourth point on my list of learning goals, the Millikan story is not well suited to illustrate the use of a plurality of methods in science as Ehrenhaft’s method did not yield the accepted results. But if several case-stories from the history of science are included in a science curriculum, it will be possible to compare methods used in different disciplines and branches of research. One could for instance compare Millikan’s method with that of
Mendeleev. Mendeleev worked theoretically on the similarities and differences between the different elements found in nature. Building on an idea put forward by the British chemist
John Newlands in 1866 he managed to structure the known element into a periodic table (in
1869).
In addition to the four learning goals explicit mentioned, I will like to mention a fifth.
Hopefully an outcome from working with the history of science in science education will be that scientific knowledge production is seen as more complicated than to follow a predefined method which automatically leads to valid and reliable knowledge. The example of Millikan might here illustrate how a researcher had to fight with his apparatus and data and using theory-laden judgements in order to be able to make nature’s answers to his experimental questions visible. Using examples from the history of science, it should be possible to illustrate both the variety of methods used, difficulties associated with experimental work, and the general difficulties in figuring out convincing accounts of natural phenomena.
Citizenship and the nature of science
So far I have argued for the history of science to be a source for illustrations in relating to aspects of the nature of science. I will now address the question of the relevance of the identified learning goals for a science curriculum for citizenship . How might historical case studies of science be of importance for citizenship today?
Narratives from the history of science provide a resource for insights and illustrations of the production of scientific knowledge. The history of science is the history of the process of research and the strives to work out reliable knowledge.
The science involved in socio-scientific issues is often scientific claims in the process of being researched, and these scientific claims are typically a focus for dispute themselves.
7
Recent examples here is whether gene modified food involves a risk to human health or to nature; whether irradiated food have reduced nutritional value; and whether the worlds change in climate is linked to human activities. Discussing reported statements of non-scientists in the media following the Chernobyl accident, Millar and Wynne (1988) concludes that there is at least as great a need for wider public understanding of the internal processes by which scientific knowledge is generated and validated as of the contents of specific areas of science. (p. 395)
They argue that the way science-based figures where used in media could develop an already widely held lay impression that science and scientific measurements is necessarily precise and accurate. This view is in stark contrast for instance to the changing predictions and advises given by scientific experts to Cumbria sheep farmers in the aftermath of the Chernobyl accident (Wynne, 1996). Examples from the history of science, like Millikan’s fight to reduce the uncertainties in his measurements, might here be used to explore the characteristics of scientific measurements and knowledge claims.
To change the underlying concept here, that science deal with facts , is of paramount importance for democratic debate as the idea of scientific facts implies that lay people have no right to doubt (Bauer, 1994 p. 63). Historical studies can be used to show that scientific claims always hinges on evaluation of different evidence, and that this evaluation is done through argumentation and criticism both in the “laboratories” and within the wider scientific community. More knowledge of this aspect of science might diminish lay people’s interpretation of disagreement between scientists in terms of interests or incompetence without further evidence for this to be the case. It might also increase lay people’s acceptance of science not being able to provide answers in short time, and thus provide the insight that decisions often must be based on other arguments then the science-based one (Collingridge &
Reeve, 1986).
Cultural frameworks
Having discussed science for citizenship and a place for the history of science in relation to the nature of science, I will now turn to discuss these issues in relation to science content learning . Thus I makes a shift from focusing on the processes of science, to a focus on the products of science.
I will maintain that knowledge of major scientific theories are highly relevant for citizenship and lay people’s ability to engage in public debates. In a Norwegian journal focusing on cultural and societal issues, the editor made the following statement discussing the relations between science and cultural issues: “To be ignorant of the works of Darwin involves an educational flaw at least as huge as not to know essential features of [the works of] Marx and Freud” (Eriksen, 1997, my translation). (Original text: “Å være uvitende om
Darwins verk er en minst like stor dannelsesbrist som ikke å kjenne grunntrekk hos Marx og
Freud”.) He points towards four central cultural issues were Darwin’s theory has an important impact: The relationship between competition and collaboration; man’s free will; the idea that the world evolves; and the relation between the individual, the art and the ecosystem. These issues do not relate directly to decision-making situations. However, while concerning our beliefs about the basic characteristics of man and our place in nature, these issues probably have a great impact on our opinions and decisions.
The theory of Darwin is not the only scientific theory of such relevance. In physics we have Kepler and his theory of the solar system and we have the so called “standard theory” of the evolution of the universe, which in fact states “when it all begun”. In chemistry we have the idea that all matter is made of atoms and molecules and while informing scholarly medicine seems to be at odds with i.e. homeopathic medicine. People unaware of these
8
“science stories” lacks the cultural frameworks needed to understand and engage in debates on a range of issues of relevance to civic life.
The mentioned lists of issues indicate that a science curriculum for citizenship should focus on the theories that are believed to have an impact on our general thinking. Millar
(1998) have argued that science education for all should focus on a few “powerful models” in science, and not try to cover all theories in all branches of science. In a report from Britain on science curriculum for the future it was argued that “a healthy democracy requires ... [a public] with a broad understanding of major scientific ideas,...” (Millar et al., 1998 p. 20). In their report they suggests an increased focus on what they call “explanatory stories”, and they suggest that “science education should make much grater use of one of the world’s most powerful and pervasive ways of communicating ideas – the narrative form...” (Millar &
Osborne, 1998 p. 13).
A science curriculum which emphasis “explanatory stories” communicated through narratives will be well suited for the inclusion of historical case studies, which also the mentioned report suggests. Such case studies ought to have a broader focus than the Millikan story however. As an example a story might include both the historical development of the atomic theory and the exploration of the structure of the atom. Importantly however, is that the historical narratives not focuses on science content alone. Inclusion of cultural aspects is needed to make it easier for the learners to comprehend links to more current debates.
Science
– society – technology interactions
My third argument on potential advantages of an inclusion of history of science concerns knowledge about the relationships between science, society and technology . In doing this I shift from a focus on the processes and products of science, to include also institutional aspects.
The inclusion of science – society interactions and the relationship between science and technology have been important aims for many science education reforms and curriculum initiatives the last decades (DeBoer, 1991; Solomon & Aikenhead, 1994; Ziman, 1980).
Science – society interactions concern questions related to characteristics of the impact of scientific knowledge and research upon the rest of society and the effect cultural, societal and political situations might have on scientific research and discoveries. Should for instance scientists decide on what energy types Norway should use? And does the surrounding culture and scientists personal philosophies influence the content of their discoveries? And how might scientific research relate to interests and power? Such questions, while being related to the process of scientific knowledge production, also relate to political and institutional aspects of science.
Such mutual influences between science and society might be illustrated by the use of historical case studies. Science – society interactions concern abstract topics, making the use of illustrations paramount. Historical case studies have at least two advantages in this respect:
First, they represent “ended stories”, making it possible to study all aspects of such interactions. Second, their political sensitivity have usually diminished, making them easier to analyse from a diversity of perspectives, and also easier to include in a science curriculum.
Interactions between science and technology might also be studied through examples from history. Technology studies indicate strongly that the view of technology merely as applied science is simply wrong (Layton, 1991). The history of the development of the Diesel engine is an interesting example here. Diesel tried to apply the scientific theory describing how a maximum efficient engine should work. Such engines should follow the so-called
Carnot cycle. However, after years of hard engineering work Diesel and his collaborators came up not with a Carnot machine, but as we all know, with a Diesel engine. In addition, progress in science often hinges on new technology made available. In modern “techno-
9
science” scientific and technological progress is often closely interconnected. The possibility of using historical case studies as a resource for teaching about science – technology interactions is worth to explore further. It is my belief that historical examples have a potential of influencing the learner’s view considerably more that a more general theoretical treatment of such issues.
Warning signs
Having explored three arguments for the inclusion on the history of science, I will try to put down a few notes of warning. The two main points concerns the question of which perspective on the history of science we want the narratives to involve, and what picture of science will emerge from the learners engagement with the stories selected.
Choice of perspective
All stories might be told in several different ways, with different emphasises and from different perspectives. In my introductory example, I tried to exemplify two different ways of telling the Millikan story. The first of these was an account of Millikan’s famous oil drop experiment, including a comment stating that Millikan demonstrated the charge of the electron to be quantified. In this slant of the story the context was minimised. Millikan is portrayed as a genial scientist working alone in his laboratory, discovering the secrets of nature. The second account of the Millikan story focused on the “Battle” between to competing groups of researchers, building on different philosophical and physical assumptions. That version also focused on Millikan’s struggle to get accurate and convincing results, and on the interplay between theory and observations.
Different choices of depth and perspective must be supposed to have a decisive impact on the learning outcome. The simplified version of the Millikan story involves a realist ontology and naive positivist epistemology. Individualist slants, while being common in science textbooks, often under-emphasises the collective and institutional dimension of science
(Ziman, 1980). A superficial treatment of the history of science might easily result in
“fictionalized idealisations” (Monk & Osborne, 1997 p. 406) where currently accepted views are seen as the most high-grade, leaving little recognition and respect for the creativity of scientists of the past.
Ziman (1980 chap. 7) have argued that to be able to appreciate interpretations of the social role of science in history students needs an adequate framework of factual knowledge.
While this problem might be dealt with through the inclusion of history of science, we are still left with the problem of selecting an adequate depth. The problem might again be illustrated by the Millikan story. In my versions I did not mention that the term “electron” was introduced by Stoney in 1891 based on his work on electrolysis. Neither did I mention that
Thomson in 1897 were able to demonstrate that the so called cathode rays were composed of negatively charged particles (He demonstrated that they were travelling with velocities much less than that of light. His results were important as they to a large extent decided the controversy regarding the existence of the electron.) Millikan definitely was in dept to these two researchers, but they again also “stood at the shoulders of giants” as Isaac Newton put it.
Ziman (1980 chap.7) also warns that the inclusion of long forgotten names and a forest of details might be uninteresting to others then professional historians of science.
Picture of science
The history of science stretches over more then thousand years. The question “Which stories of science?” therefor also involves choices related to historic periods. Are narratives from ancient Greece and medieval science relevant, or should one focus on the Renaissance, the
10
Enlightenment period or the nineteenth century? Will the picture that emerges about the nature of science from these different periods be the same? Of course not!
Science is an institution that has changed radically throughout history. Science today differs dramatically from the science of the sixteenth or eighteenth century. Following the institutionalisation of natural philosophy in the seventeenth century and the professionalisation of science in the nineteenth century, the twentieth century gave us the socialisation and industrialisation of science (Aikenhead, 1994). Science’ integration with industry and technology makes the older pictures of science as “the disinterested pursuit of objective truth” (Jenkins, 1992 p. 232) untenable. The role of scientific expertise in many socio-scientific issues also illustrates the socialisation of science. In such issues scientific experts very often represents governmental agencies, industry, environmental groups or other interests, and can hardly be assumed to represent neutral perspectives.
The need for science education to reflect this change in the nature of science has been argued strongly by several science educators (Aikenhead, 1994; Jenkins, 1992; Osborne,
1997). Discussing the historical evolution of both science and science education, Aikenhead
(1994) ask the pertinent question “How much longer can 19 masquerade as legitimate science?” (p. 20). th
century school science
If Galileo Galilei (1564-1642), Carl von Linné (1707-1778), Charles Darwin (1809-1882) and Albert Einstein (1879-1955) represents different kinds of science (Jenkins, 1992), then the inclusion of historical case-stories in a science curriculum for citizenship need to be done with great care. It is paramount that a science curriculum for citizenship communicates an adequate picture of science as practised today.
Conclusion
Throughout this talk I have been able to use the Millikan story to illustrate different arguments for the inclusion of the history of science in a science curriculum for citizenship.
Undoubtedly the history of science represents a rich reservoir of examples and illustrations suitable for the teaching of the nature of science and science – society – technology interactions. It also offers a possibility to contextualise and humanise (Irwin, 2000; Thomsen,
1998) the teaching of “explanatory stories”. I believe, however, that studying historical case stories in addition to serve to illustrate also have the potential of convey depth in the learner’s understanding of these different topics.
But as I have tried to argue in the last part of this lecture, there are some pitfalls one need to avoid when including the history of science in a science curriculum for citizenship. We need to balance between superficial treatment and boring depth, and we need to choose perspectives consciously. I believe the selection ought to be done on the basis of explicitly stated learning goals. In this selection we should also be aware of the possibility of choosing cases showing that also women and non-white people have contributed to the growth of science.
Most importantly, however, is the balance between historical and contemporary case studies. This is essential for a science curriculum for citizenship . One approach could be to use historical studies to gain a perspective on modern industrialised and socialised science.
But if case studies on contemporary science are expelled from a curriculum, my advice would be not to include historical studies either.
References
Aikenhead, G. S. (1987). High School Graduates' Beliefs about Science-Technology-Society.
III: Characteristics and Limitations of Science Knowledge. Science Education, 71 (4),
459-487.
11
Aikenhead, G. S. (1994). The Social Contract of Science. In J. Solomon & G. Aikenhead
(Eds.), STS Education. International Perspectives on Reform (pp. 11-20). New York:
Teachers College Press.
Arons, A. B. (1988). Historical and philisophical perspectives attainable in introductory physics courses. Educational Philosophy and Theory, 20 (2), 13-23.
Bauer, H. H. (1994). Scientific Literacy and the Myth of the Scientific Method . Urbana,
Illinois: University of Illinois Press.
Bernal, J. D. (1978). Vitenskapens historie (The history of science) ( Vol. 3). Oslo: Pax forlag.
Collingridge, D., & Reeve, C. (1986). Science Speaks to Power: the Role of Experts in Policy
Making . London: Frances Pinter.
DeBoer, G. E. (1991). A History of Ideas in Science Education. Implications for Practice .
New York: Teachers College Press.
Driver, R., Leach, J., Millar, R., & Scott, P. (1996). Young Peoples' Images of Science .
Buckingham: Open University Press.
Eriksen, T. H. (1997). Vitenskapen og kulturlivet (Science and cultural issues). Samtiden .
Fullinwider, R. K. (1987). Technological literacy and citizenship. Bulletin of Science,
Technology and Society, 7 , 320-324.
Holton, G. (1978). The Scientific Imagination: Case Studies . Cambridge: Cambridge
University Press.
Holton, G. (1995). Einstein, History, and Other Passions . New York: AIP Press.
Irwin, A. R. (2000). Historical case studies: teaching the nature of science in context. Science
Education, 84 , 5-26.
James, E., Eijkelhof, H., Gaskell, J., Olson, J., Raizen, S., & Sáez, M. (1997). Innovations in science, mathematics and technology education. Journal of Curriculum Studies, 29 (4),
471-483.
Jenkins, E. W. (1992). School science education: towards a reconstruction. Journal of
Curriculum Studies, 24 (3), 229-246.
Layton, D. (1991). Science education and praxis: the relationship of school science to practical action. Studies in Science Education, 19 , 43-79.
Millar, R. (1996). Towards a science curriculum for public understanding. School Science
Review, 77 (280), 7-18.
Millar, R., & Osborne, J. (1998). Beyond 2000: Science Education for the Future . London:
Nuffield Seminar Series: Interim Report V3.
Millar, R., Osborne, J., & Nott, M. (1998). Science education for the future. School Science
Review, 80 (291), 19-25.
Millar, R., & Wynne, B. (1988). Public understanding of science: from contents to processes.
International Journal of Science Education, 10 (4), 388-398.
Millikan, R. A. (1951). The Autobiography of Robert A. Millikan.
London: Macdonald & Co.
Monk, M., & Osborne, J. (1997). Placing the history and philosophy of science on the curriculum: a model for the development of pedagogy. Science Education, 81 , 405-
424.
Osborne, J. (1997, September). Science education for the future - The road ahead?
Paper presented at the First International Conference of the European Science Education
Research Association, Rome.
Shamos, M. H. (1995). The Myth of Scientific Literacy . New Brunswick: Rutgers University
Press.
Sjøberg, S. (1997). Scientific literacy and school science. Arguments and second thoughts. In
E. Kallerud & S. Sjøberg (Eds.),
Science, Technology and Citizenship. The Public
Understanding of Science and Technology in Science Education and Research Policy
(pp. 9-28). Oslo: Norwegian Institute for Studies in Research and Higher Education.
12
Solomon, J., & Aikenhead, G. (Eds.). (1994). STS Education. International Perspectives on
Reform . New York: Teachers College Press.
Thomsen, P. V. (1998). The historical-philisophical dimension in physics teaching: Danish experiences. Science & Education, 7 , 493-503.
Wynne, B. (1996). Misunderstood misunderstandings: social identities and public uptake of science. In A. Irwin & B. Wynne (Eds.), Misunderstanding Science? The Public
Reconstruction of Science and Technology (pp. 19-46). Cambridge: Cambridge
University Press.
Ziman, J. (1980). Teaching and Learning About Science and Society . London: Cambridge
University Press.
Øgrim, Ormestad, Lunde, & Jerstad. (1985).
Rom Stoff Tid 3. Fysikk 3 Fy (Space, Matter,
Time. Physics thirteenth grade) . Oslo: J. W. Cappelens Forlag.
13