Development of a Rapid Test for Ebola Virus Infection by Integrated
advertisement

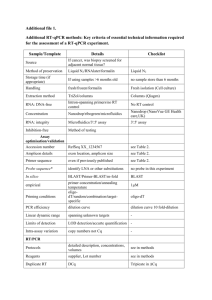
Development of a Rapid Test for Ebola Virus Infection by Integrated Comprehensive Droplet Digital Detection Thi N. Nguyen, Abraham Phung Mentors: Dong-Ku Kang, Weian Zhao The largest outbreak in history of Ebola hemorrhagic fever (EHF), caused by an infection of a deadly virus, occurred in West Africa in 2014. The capability to rapidly detect EHF in the early stage of the infection is critical to enhance effectiveness of treatments and to contain the spread of the disease. Current clinical diagnoses use antigen-capture ELISA and reverse transcription PCR (RT-PCR) to detect the virus. However, these tests are only effective several days after the patient displays symptoms, while severe symptoms increase during the 2 to 21 days after viral invasion. To address this need, we are developing an assay based on the Integrated Comprehensive Droplet Digital Detection system (IC3D), which can rapidly and sensitively detect Ebola viral RNA from patients’ blood within 3.5 hours with minimum sample preparation. The assay includes: (1) quantitative reverse transcription PCR (RT-qPCR) targeting a viral RNA constructs of the nucleoprotein (NP) region within the Ebola genome, (2) droplet based-microfluidics that encapsulates target RNA with RT-PCR reagents within picoliter-volume droplets, and (3) high-throughput digital droplet counting with a particle counter. We optimized the RT-qPCR conditions for bulk RT-qPCR with the annealing temperature at 58 for 1 minute for 40 cycles (other steps are based on a commercial kit of one-step ddRT-PCR mixture) to allow for maximum target RNA amplification within droplets. Successful development of our assay will provide the tool for rapid and sensitive quantification of Ebola RNA in a milliliter of human plasma.