Homework 3
advertisement
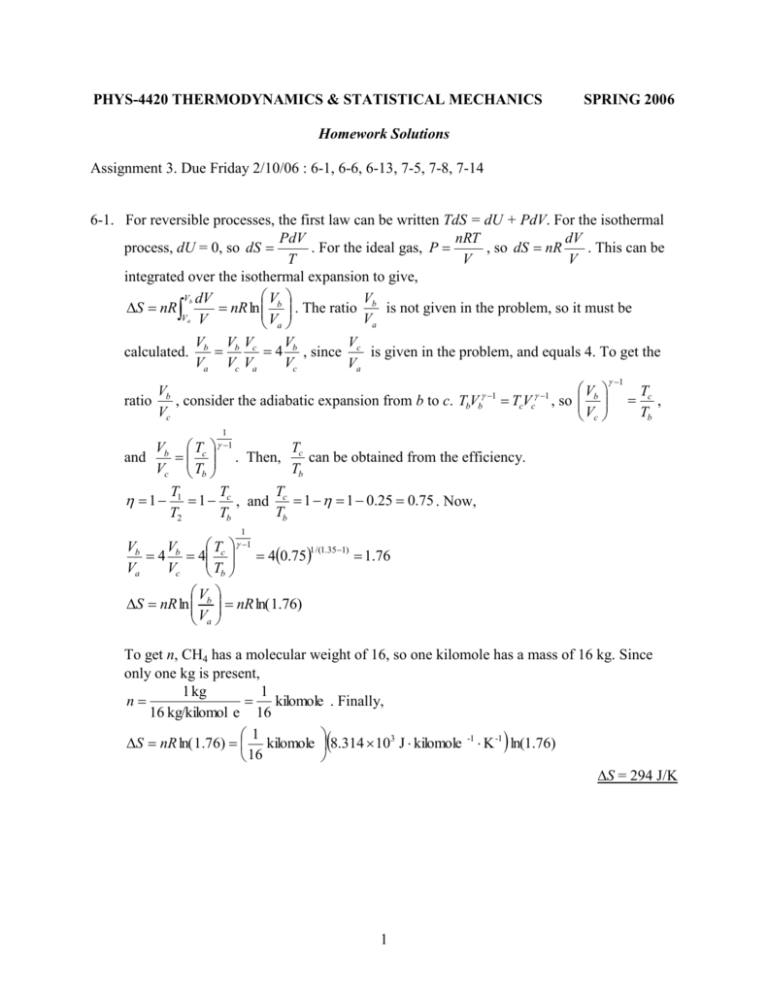
PHYS-4420 THERMODYNAMICS & STATISTICAL MECHANICS SPRING 2006 Homework Solutions Assignment 3. Due Friday 2/10/06 : 6-1, 6-6, 6-13, 7-5, 7-8, 7-14 6-1. For reversible processes, the first law can be written TdS = dU + PdV. For the isothermal PdV nRT dV process, dU = 0, so dS . For the ideal gas, P , so dS nR . This can be T V V integrated over the isothermal expansion to give, Vb dV V V S nR nR ln b . The ratio b is not given in the problem, so it must be Va V Va Va calculated. Vb Vb Vc V V 4 b , since c is given in the problem, and equals 4. To get the Va Vc Va Vc Va V ratio b , consider the adiabatic expansion from b to c. TbVb 1 TcVc 1 , so Vc Vb Vc 1 Tc , Tb 1 T Vb Tc . Then, c can be obtained from the efficiency. Tb Vc Tb T T T 1 1 1 c , and c 1 1 0.25 0.75 . Now, Tb T2 Tb and 1 1 T 1 Vb V 1 /(1.35 1) 4 b 4 c 40.75 1.76 Va Vc Tb V S nR ln b nR ln( 1.76) Va To get n, CH4 has a molecular weight of 16, so one kilomole has a mass of 16 kg. Since only one kg is present, 1 kg 1 n kilomole . Finally, 16 kg/kilomol e 16 1 S nR ln( 1.76) kilomole 8.314 103 J kilomole -1 K -1 ln(1.76) 16 S = 294 J/K 1 6-6. There are several things that could be checked. Only the second or third one has to be shown to get credit. i) Does this violate the first law? Does it “create” energy? Check if |W| > |Q2| – |Q1|. |W| = (15 kw·hr)(103 w/kw)(3600 s/hr) = 5.4 ×107 J |Q2| – |Q1| = 10 ×107 J – 4 ×107 J = 6 ×107 J > 5.4 ×107 J. No violation. ii) Does this violate the second law? Does it decrease the entropy of the universe? At the high temperature reservoir, |Q2| is transferred into the engine at temperature T2. Q2 1 108 J Then, for the engine, 2.5 105 J/K . T2 400 K At the low temperature reservoir, |Q1| is transferred out of the engine at temperature T1. Q 4 107 J Then for the engine, 1 2 105 J/K . T1 200 K Q Q The sum of the two 1 2 0 which is a violation of the Clausius inequality, T1 T2 dQ T 0 , which is equivalent to a violation of the second law. iii) Is the engine more efficient than a Carnot engine? W 5.4 107 J 0.54 54% Q2 10 107 J T1 200 K 1 0.50 50% T2 400 K > Carnot . Something is wrong. Do not invest! Carnot 1 2 6-13. Path 1→2 is adiabatic, so S1→2 = 0 T cP dT 5 T dT 5 2 R 2 R ln 3 T T T T T2 T c dT T dT T Path 3→1 is isochoric, so S31 v 32 R 32 R ln 1 T T T T T3 Path 2→3 is isobaric, so S23 T3 3 2 2 1 1 3 3 To complete the problem, temperatures T1, T2 and T3 can be calculated. P v (10 atm)(1.01 105 Pa/atm)(2 m3 ) For T1, T1 1 1 243 K R 8.31 103 J/K 1 v For T2, T1v1 1 T2v2 1 , so T2 T1 1 v2 2 m3 243 K 3 4m 5 1 3 153 K v Pv Pv For T3, T3 3 3 2 1 , and P1v1 P2v2 , so P2 P1 1 . Then, R R v2 5 3 2 m3 v v Pv v v 76.5 K T3 P1 1 1 1 1 1 T1 1 243 K 3 R v2 v2 R v2 4m T 76.5 K 4 S23 52 R ln 3 52 (8.31 103 J/K)ln 1.44 10 J/K 153 K T2 T 243 K 4 S31 32 R ln 1 32 (8.31 103 J/K)ln 1.44 10 J/K 76.5 K T3 For the entire cycle, S = S1→2 +S2→3 +S3→1 = 0 – 1.44 ×104 J/K + 1.44 ×104 J/K = 0 Alternate approach: For 2→3, P is constant, so T v. Then, T3 S2 3 T2 T3 v3 1 so T2 v2 2 T dT T cP dT 1 cP cP ln 3 cP ln cP ln 2 T T T 2 T2 3 2 T P P v For 3→1, v is constant, so T P. Then, 1 1 1 2 2 so T3 P3 P2 v1 T1 c dT T1 dT c T S31 v cv cv ln 1 cv ln 2 cv ln 2 cv p ln 2 c p ln 2 T3 T T3 T cv T3 For the entire cycle, S = S1→2 +S2→3 +S3→1 = 0 – cp ln 2 + cp ln 2 = 0 3 7-5. a) To calculate the entropy change of the gas, use a reversible process that takes the gas from the same initial to the same final state. An isothermal expansion can be used. Pdv RT dv dv R Tds = dU + Pdv. For the isothermal process, dU = 0, so ds T v T v 3v0 dv 3v s = 9.13 ×103 J/K s R R ln 0 R ln 3 (8.31 103 J/K)ln3 v0 v v0 b) Since this is a free expansion, the gas is isolated, and the surrounding undergo no entropy change. Therefore, the entropy change of the universe is the same as the entropy change of the gas. suniverse = 9.13 ×103 J/K dQ mcP dT . T T Then, the expression can be integrated from the initial to the final temperature (Tf) for each body, and the results added. That gives, T 2 T f2 Tf T dT T f T dT mcP ln f S mcP mcP ln ln mcP ln T T T T T T T2 T1 T1T2 1 2 T S 2mcP ln f . All that remains is to find Tf. The heat that flows from the TT 1 2 hotter body all goes to the cooler body, so Q1 + Q2 = 0, or T T mcP(Tf – T1) + mcP(Tf – T2) =0. Then, T f 1 2 . When this is put into the equation 2 T T for S, the result is: S 2mcP ln 1 2 2 TT 1 2 7-8. a) The entropy change for one of the bodies can be calculated from dS f 1 f 2 T1 T2 1 , or T1 T2 2 T1T2 . To prove that this is 2 T1T2 true, begin by squaring both sides. Since there are no negative quantities involved, if the square of the left side is greater than the square of the right side, the proof is done. T12 T22 2T1T2 4T1T2 . Now subtract 4T1T2 from both sides to give, T12 T22 2T1T2 0 , or (T1 – T2)2 > 0. This is true, except when T1 = T2, but in that case there would be no heat flow, and no entropy change, so the result would still be correct. b) To have S > 0, we must have 4 v v dv dT dP . Then, T P P T dv 1 v 1 v 1 v 1 v dT dP dT kdP , since and k v v T P v P T v T P v P T dv Since is an exact differential, it is the differential of some function f(T, P) = ln v. v f f f f df dT dP dT kdP . Then, and k . T P P T T P P T 2 f 2 f Since df is an exact differential, . Therefore, PT TP P T T P 7-14. v = v(T, P), so 5