Dear Liver EQA Member, - Virtual Pathology at the University of Leeds
advertisement

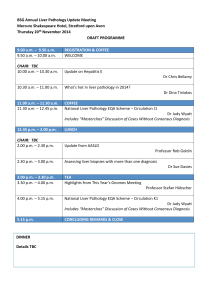
22.01.07 Dear Liver EQA Member, Circulation U: Circulation U is due to complete on 23rd February – please make sure you have sent Anne your responses by that date. The meeting to discuss the cases is during the BSG meeting and will be on Tuesday 27th March at 12.30 – 2pm in the Jura Room of the Crown Plaza Hotel. This hotel adjoins the Scottish Exhibition and Conference Centres. There will be a buffet lunch provided. Please let Anne know whether you anticipate being able to attend. Tuesday is the BSG day with liver presentations – the programme is available on http://www.bsg.org.uk/pdf_word_docs/meeting_07.doc Circulation V: This starts on February 16th and ends on June 8th, and will be discussed on Friday July 6th, 2007, during the joint meeting of the Pathological Society/BDIAP in Glasgow. This follows a GI symposium 9-12 that morning. The Liver EQA meeting will be in lecture theatre A in the Boyd Orr building of Glasgow University. Liver Update Meeting. The update meeting in Nottingham last November was well received, and feedback comments gave support for this becoming a regular event. Three quarters of you who came to the liver meeting were also at the oesophageal meeting on the previous day. This year, therefore, we are planning a similar arrangement, with the liver update meeting in Lancaster on Thursday 6th December, followed by a ‘hollow’ GI meeting arranged by Nic Mapstone, on Friday 7th December, where the programme will be focussing on MDT meetings. CPA accreditation: I have had discussions with Eddy Walsh from CPA – I am not working on reaccreditation for the Liver EQA scheme yet, because accreditation of the scheme depends on the hosting laboratory being accredited – currently work in progress in Leeds. Many EQA schemes are not continuing with CPA accreditation since its new regulations - determined by international standards for laboratory accreditation – seem to be inappropriate for purposes of EQA schemes in histopathology. Nevertheless, Anne and I will be updating the documents for the scheme, and ensuring we comply with the relevant good practice components of the CPA standards. Health Resource Groups (HRGs) Attached to this email are documents relating to HRGs. These were circulated following the Histopathology Sub-Specialists meeting at RCPath in November. Please take time to open and read them. In order to feed into the determination of realistic tariffs for histopathology we are asked to collect data on the workload – in terms of blocks, slides, and pathologist’s time – entailed in reporting histopathology cases, including discussions in MDT/CPC meetings (see action required, page 1-2 in the Advisors’ covering letter attached). There is a balance to be struck between properly recognising the work involved and avoiding an unrealistic degree of complexity in data handling. At this stage, if you have and audit data relating to this, or would be interested in collecting some, please will you let me know on the reply slip of this email. It will be important to have evidence supporting low volume/high complexity work such as liver pathology. I understand that histopathology is no longer part of HRG4, and so the timetable for this work is less tight than originally thought; we are still asked to press on with this information gathering phase. Liver Histopathology Organisation As the organiser of the liver EQA scheme I was invited to be the RCPath sub specialist representative for liver histopathology when this was created in 2005 – liver was previously included within GI pathology. Working on the cancer dataset, tissue pathways, and now HRG initiatives has demonstrated the need for a properly recognised group to ensure that we can maintain high quality liver pathology services in the future. Clinical provision is towards increasing development of local hepatologist expertise. A recognition of the currently informal network arrangements for supporting local interested histopathologists with central review of biopsies where there are clinical or histological indications will be required. This proposed group could oversee such activities as CPD, EQA, coordination with clinical developments, advice on service provision, and potentially national research initiatives, e.g. in primary liver cancers. It should include non-tertiary pathologist and clinician representation. Administratively, this may be best achieved through a liver subcommittee of the pathology section of the BSG, and this will be discussed at its next meeting in March. Liver is represented by Alastair Burt and (from April) by Stefan Hubscher. I anticipate there will be time to discuss and develop these ideas at the EQA meeting in July. I plan to circulate a questionnaire about the EQA scheme and these developments before that meeting. Apologies for such a long letter – for now please send in your circulation U responses as soon as you have them, and reply to Anne’s email message to which this letter is attached, With best wishes, Judy Wyatt Organiser, National Liver Histopathology EQA Scheme.