SNC_02_08-10_Properties of Common Substances Lab
advertisement

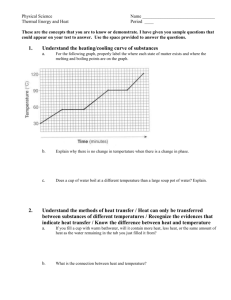
Properties of Common Substances Lab Purpose: Part A: To determine the identity of common substances using physical properties. Part B: To observe various materials and classify the matter. Materials: Part A: Substances A - D Hot plate Petri dish Conductivity tester Beakers Magnifying glass Magnets Well plates Scoopula Distilled Water Pipettes and bulbs Tongs Part B: Salt Sucrose Cobalt (II) chloride Sand Water Hydrogen gas Air Soil Rocks Jars with Lids Iron (III) chloride Milk Poweraid 16 mile creek water Beakers Vials with Lids Safety: Part A: • Read over the MSDS for all of the substances you will be using in this experiment. • Create a table to record the physical properties for each of the substances A – D that you feel could help you distinguish between the substances (i.e. melting point, colour, appearance, etc.) when you actually do the experiments.. • Do NOT taste, smell, or handle any of the substances. Part B: • Read over the MSDS for all of the substances you will be using in the experiment. • Do NOT point the laser at people. Procedure: Part A: 1. Create a table in the observations section of your lab report with the following headings; Sample, Qualitative Observations, Melting Point, etc. 2. Collect a small sample of each of the chemicals used for this experiment. Be sure to keep them separate and labelled so that you know which substance is which. 3. Take qualitative observations of each of the samples. Do NOT taste or smell the substances. 4. Conduct any other tests that you feel would provide you with enough information to determine the identity of the unknown substances. The instructions for each test are on the lab benches. 5. In order to be sure that you have identified the substance correctly when you do your analysis you need to confirm at least two physical properties. Note: for melting points you will not have an exact temperature for comparison but the relative temperature should help you determine the identity. Part B: 1. Create a table with the following headings; Station #, Substance(s) Present, Classification. 2. Move through each of the 10 stations and classify the matter using the terminology discussed in class. Analysis: Complete the following questions in your lab report. Part A: 1. Determine the identity of each of the substances A – D and provide an explanation for each. Hint: you should discuss the physical properties that led you to determine the identity. [12 Inquiry] 2. Why is it not safe to smell the solids? [2 Application] 3. If you were colour blind how could you determine the difference between copper (II) sulfate pentahydrate and sucrose? [2 Application] Part B: 1. Which substance did you find the most difficult to classify? Explain. [2 Application] 2. The name tag fell off of a container containing a yellow substance (all of the particles in the container look the same) what classification would you give the unknown substance? Explain your reasoning. [2 Application] Conclusion: Part A: Restate the purpose and summarize your findings. Part B: No conclusion necessary. Evaluation: You will be given time to write up your lab report in class. Do not take your lab home it needs to be submitted at the end of each period. The write up for your lab report will be discussed in class. There are certain sections that you can say refer to handout “Properties of Common Substances Lab” when you write up your report. The set up of your tables as well as the format of your lab report will be Communication marks. Your lab report will also be marked for Inquiry and Application as stated in the analysis section of this handout. Learning Skills: During the course of the lab you will be assessed on the following learning skills. Learning Skill Learning Skill “Look Fors” Level Responsibility Fulfils responsibilities and commitments within the learning environment. N S G E Organization Identifies, gathers, evaluates, and uses information, and resources to complete tasks. N S G E Independent Work Uses class time appropriately to complete tasks. Follows instructions with minimal prompting. N S G E Collaboration Accepts various roles and an equitable share of work in a group. N S G E Initiative Approaches new tasks with a positive attitude. N S G E Self-Regulation Seeks clarification or assistance when needed. N S G E