Chapter 5
advertisement

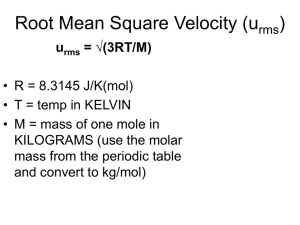
Chapter 5 (Gases) Outline 5.1 Pressure and Units of Pressure A. Pressure – force exerted over a given area (pressure = force/area) 1. device used to measure atmospheric pressure: the barometer – invented by Torricelli B. units of pressure – since instruments used for measuring pressure often contain mercury, “the most commonly use units for pressure are based on the height of the mercury column: mm Hg (millimeter of mercury); often called the torr because of Torricelli 1. 2. 3. 4. mm Hg torr standard atmosphere (atm) pascal (Pa – the unit used in the SI system – stands for newtons per meter squared) * I atm = 760 mm Hg = 760 torr = 101325 Pa * 5.2 The Gas Laws A. Boyle’s Law (Robert Boyle) P1V1 = P2V2 (P = pressure, V = volume) - a gas that strictly obeys Boyle’s Law is an ideal gas B. Charles’s Law V1/T1 = V2/T2 (T = temperature in Kelvin) - K = degree Celsius + 273 - Zero K is called absolute zero C. Avogadro’s Law V1/n1 = V2/n2 (n = number of moles of gas particles) D. Gay – Lussac’s Law P1/T1 = P2/T2 E. Combined Gas Law P1V1/T1 = P2V2/T2 5.3 The Ideal Gas Law A. PV=nRT B. R = the universal gas constant (0.08206 L atm/K mol) C. the ideal gas equation “expresses behavior that real gases approach at low pressures and high temperatures”; therefore, an ideal gas is a hypothetical substance 5.4 Gas Stoichiometry A. molar volume of an ideal gas = 22.4 liters (at 0 degree Celcius and 1 atm; aka standard temperature and pressure = STP) 5.5 Dalton’s Law of Partial Pressures A. “For a mixture of gases in a container, the total pressure exerted is the sum of the pressures that each gas would exert if it were alone” Ptotal = P1 + P2 + P3… (each P represents a partial pressure) B. mole fraction – “the ratio of the number of moles of a given component in a mixture to the total number of moles in the mixture” X1 = n1/ ntotal - the mole fraction can be expressed in terms of pressure X1 = n1/ ntotal = P1/Ptotal C. vapor pressure of water – the pressure of the vapor over water D. Kinetic energy = 3/2 RT 5.6 The Kinetic Molecular Theory of Gases A. kinetic molecular theory (KMT) – ideal gasses 1. “particles are so small compared with the distances between them that the volume of the individual particles can be assumed to be negligible” 2. “The particles are in constant motion. The collisions of the particles with the walls of the container are the cause of the pressure exerted by the gas.” 3. “the particles are assumed to exert no forces on each other; they are assumed neither to attract or repel” 4. “the avg. kinetic energy of a collection of gas particles is assumed to be directly proportional to the Kelvin temp. of the gas” B. root mean square velocity (u rms)– avg. of the particle velocities mol) u rms = square root of 3RT/M (M = mass of a mole of gas particles in kilograms; R = 8.3145 J/K B. real gases – have volume; exert forces of attraction and repulsion on each other; transfer K.E. during collisions 5.7 Effusion and Diffusion A. diffusion – the mixing of gases B. effusion – the passage of a gas through a tiny space into an evacuated chamber 1. Graham’s law of effusion: rate of effusion for gas 1/ rate of effusion for gas 2 = square root of M2/ square root of M1 (M represent molar masses of the gases) 5.8 Real Gases Pobs = nRT/(V-nb) – a(n/V)2 (Pobs = observed pressure; V = volume of the container; nb = volume correction; a(n/V)2 = pressure correction) 5.9 Chemistry in the Atmosphere A. two main sources of air pollution – transportation, production of electricity (ex. through burning coal) 1. much of the coal in the Midwest contains sulfur; through a series of reactions, the sulfur ends up in sulfuric aid, which results in acid rain Multiple Choice Questions: 1. Oxygen, which is 16 times as dense as hydrogen, diffuses (A) 1/16 as fast (B) ¼ as fast (C) 4 times as fast (D) 16 times as fast 2. Under which conditions will a gas behave most ideally? (A) low P and high T (B) high P and low T (C) low P and low T (D) high P and high T 3. Which pair of gases has the same average rate of diffusion at 25 degrees Celsius? (A) He and Ne (B) N2 and O2 (C) N20 and CO2 (D) NH3 and HCl 4. A sample of neon gas has a volume of 248 mL at 30. degrees Celsius and a certain pressure. What volume would it occupy if it were heated to 60. degrees Celsius at the same pressure? (A) 226 mL (B) 273 mL (C) 278 mL (D) 496 mL 5. A gas has a volume of 6.0 L at a pressure of 0.80 atm. What is the volume if the pressure is changed to 0.20 atm at constant temperature? (A) 1.5 L (B) 3.0 L (C) 12 L (D) 24 L Free Response: A student collected a sample of hydrogen gas by the displacement of water. The relevant data are: Volume of sample: 90.0 mL Temperature: 25 degrees Celsius Atmospheric pressure: 745 mm Hg Equilibrium Vapor Pressure of H20 (25 degrees Celsius): 23.8 mmHg (a) Calculate the number of moles of hydrogen gas collected. (b) Calculate the number of molecules of water vapor in the sample gas. (c) Calculate the ratio of the avg. speed of the hydrogen molecules to the average speed of the water vapor molecules in the sample. (d) Which of the two gases, H2 or H20, deviates more from ideal behavior? Explain your answer. Answers: Multiple Choice 1. (b) rate1/rate2 = (square root of M2)/ (square root of M1) = (square root of 1.01)/ (square root of 16.00) =¼ 2. (a) (conceptual – low P will allow more space for gas molecules, and so their sizes would be negligible when compared to the distance between them; high T will allow the gas molecules to move faster, so they will exert less force on each other) 3. (c) rate1/ rate 2 = (square root of M2) / (square root of M1) M = molar mass if rate 1 = rate 2, then M1 = M2 N2O molar mass 14.01 x 2 16.00 = 44.02 (g/mol) CO2 molar mass 12.01 32.00 = 44.01 (g/mol) 44.02 is about 44.01 4. (b) P1V1/T1 = P2V2/T2 P is constant 248 mL / 303 K = x mL / 333 K x = 273 mL 5. (d) P1V1 = P2V2 (0.80 atm)(6.0 L) = (0.20 atm)( V2) V2 = 24 L Free Response – (a) Patm = PH2 + PH2O PH2 = (745 – 23.8)mmHg = 721.2 mmHg x (1 atm / 760 mmHg) PH2 = 0.9499 which is about 0.950 atm n = PV/RT = (0.949 atm)(0.090 L) / (0.08206)(25 + 273 K) nH2 = 0.00349 mol H2 (b) nH2O = PV/RT = [(23.8 mmHg x 1 atm/ 760 mmHg)(0.090 L )] divided by (0.08206)(25 + 273 K) nH2O = 0.000115 0.000115 mol H20 x (6.022X1023 H2O molecules) / (1 mol H2O) = 6.93 x 1019 molecules of H2O (c) rate H2 / rate H20 = (square root of 18.02 g) / (square root of 2.02 g) = 2.99 (d) H20 will deviate more from ideal behavior because it has a larger volume, and it has stronger intermolecular forces because it is a polar molecule (and H – bonds)