CH107 College Chemistry
advertisement

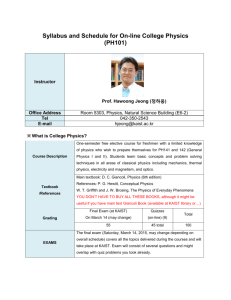
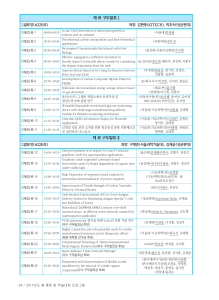
CH107 College Chemistry Syllabus, Fall 2009 Instructor: Prof. Alan J. Buglass (042-350-2842; ajbuglass@kaist.ac.kr) Teaching Assistants: Kim Kibong (042-350-2885; kkb826@kaist.ac.kr), Chong Tae Hueng (042-350-2885; jiodorumi@kaist.ac.kr), Kang Ho Seok (042-350-2873; hosuk@kaist.ac.kr). Textbook: L.J. Malone and T.O. Dolter, Basic Concepts of Chemistry. J. Wiley and Sons Inc. 8th Edition (International Student Version), 2009. Weekly Lecture Schedule Week Content and Comments 1 Introduction/Prologue/Ch. 1: Measurement in Chemistry. Classes start September 2. 2 Ch. 1/Ch. 2: Elements and Compounds. 3 Ch. 3: The Properties of Matter and Energy. Quiz 1 (on Ch. 1-3). 4 Ch. 4: The Periodic Table and Chemical Nomenclature/Ch. 5: Chemical Reactions. 5 Ch. 5/Ch. 6: Quantities in Chemistry. 6 Ch. 6/Ch. 7: Quantitative Relationships in Chemical Reactions. Friday, October 2 is a holiday. 7 Ch. 7. Review. Quiz 2 (on Ch. 4-7). 8 Mid-Term Exam (on Ch.1-Ch.7) 9 Ch. 8: Modern Atomic Theory. 10 Ch. 9: The Chemical Bond. 11 Ch.10: The Gaseous State. 12 Ch. 11: The Solid and Liquid States. Quiz 3 (on Ch. 8-11). 13 Ch.12: Aqueous Solutions/Ch. 13: Acids, Base, and Salts. 14 Ch. 13/Ch. 14: Oxidation-Reduction Reactions. 15 Ch. 14. Review. Quiz 4 (on Ch. 12-14). Final Exam: Week 16 (on Ch. 8-Ch. 14). Homework: This is set in the following weeks, to be submitted to your TA the week after. Week 2, 3, 4, 5, 6, 10, 11, 12, 13, 14. Grading: Mid-term exam 20%; Final Exam 30%; Quiz (20%) (total); Homework 25% (total); Attendance 5%.