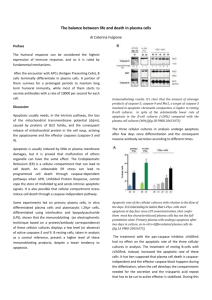
Initial observations 24 hours after cell culture preparation indicate no
advertisement

Abstract Cell Culture studies are necessary in order to investigate individual cell functions and characteristics, as well as intercellular interactions. Observations were made on the growth and subsequent tissue formation of a non-tumorogenic, transformed cell line of mammary epithelial origin, MCF-10-2A. Cell proliferation was observed in three types of media (basal, differential and growth) using a variety of substratum components (collagen IV, laminin, fibronectin and plastic). These particular cells showed no distinctions in growth patterns across the given experimental conditions. Cell death, in particular, apoptosis, is often associated with tissue formation. This phenomenon was not observed during a week of observations and was thus induced by application of Thapsigargin and Staurosporine in a dose-dependent manner. Significant apoptosis of MCF-10-2A was observed only in reaction to Staurosporine. These results indicate that this particular cell line has the ability to undergo apoptotic activity; however, the transformed nature of the cells may prevent expression of apoptosis at the level exhibited in normal mammalian cells. Introduction Cell Culture: Cell culture is an essential tool when trying to address questions that require the examination of many cells. It allows for the study of living cells that are growing under controlled conditions. Therefore, questions relating to cell function can be addressed in addition to those concerning cell structure. (1) In this study, we chose to culture and examine nontumorigenic and tumorigenic mammary gland epithelial cells. This paper focuses on the results obtained from MCF-10-2A cell cultures, a non-tumorigenic epithelial cell line (2). Although cell culture allows for work with live cells, it is still an in vitro setup. Oftentimes, cells will behave differently in vitro than they will in vivo (1). Only when placed in an appropriate environment will they undergo and maintain normal differentiation and growth (3, 4). Therefore, one of the important variables to consider when culturing cells is liquid media type. In this study, we were particularly interested in characterizing cell growth. Thus we chose three different media types: modified F12 +Basal, +Growth and +Differential. Literature review indicates that Growth and Differential media constituents should decrease the time it takes for tissue development and cell differentiation. The presence of insulin increases the uptake of glucose and galactose, which provide an essential source of energy and also serve as the building blocks for the formation of lactose (5). In comparison, Basal medium, contains only the standard 2% serum contained in all media types. While the serum provides some growth factors, lower growth rates are expected with Basal medium because it lacks insulin. Differential medium contains cortisol and prolactin as well, which work to stimulate the synthesis and secretion of lactose (5). The combination of these additional supplements should provide for an even greater increase in the density of the cell culture. In addition to specific media, cells also often require the appropriate physical environment with regard to the extracellular matrix in order to maintain specific function and exhibit optimal growth rates (1). In this study, cells were plated on four different types of substratum: collagen IV, fibronectin, laminin and plastic which served as a control. In vivo, mammary epithelial cells can be found in contact with all of the test substratum, except for plastic (6). Collagen and fibronectin are known to play a role in cell adhesion (6, 7). Whereas laminin, has been shown to help signal cell differentiation (6). The addition of these various substratum should allow for greater cell adhesion and proliferation as compared to culture on plastic alone. In this study, cell growth for all experimental conditions was monitored using phase contrast microscopy. The results were used to assess the effects of media and substratum on cell growth and tissue formation. Apoptosis: Apoptosis is a gene-dependent and energy-dependent method of cell death that plays an integral role in the proliferation and modification of various tissues in mammalian hosts. It is characterized as a programmed function due to its basis as a fixed genetic program, but can be modified or triggered due to external and environmental stimuli. Senescence via this mechanism acts to reduce excess numbers of cells that can occur during growth, differentiation and malignant transformation. This action is in contrast to Necrosis, an alternative method of cell death, both physically and functionally. Necrotic cells are distinguishable by a swell in size. Apoptotic cells are recognized by cell shrinkage and chromatin condensation (8). Thapsigargin and Staurosporine are two reagents that trigger apoptotic cell death in a variety of cell types. Thapsigargin acts as an inhibitor of endoplasmic Ca2+ and Mg2+ ATPase activity. This inhibition causes a release of Ca2+ from cytosolic stores, resulting in an influx of Ca2+ into the cytoplasm. Certain cells types, such as mesangial cells, react to this activity by inducing apoptosis. However, in other cells such as neutrophilic granulocytes, the increase in cytoplasmic Ca2+ rescues cells from an apoptotic fate (8). Application of thapsigargin in sequential concentrations will yield the effect, if any, of this specific inhibitory activity on mammary epithelial cell fate. Staurosporine is a protein kinase C inhibitor, and it exhibits similar effects on a number of cell types. Disruption of cyclin dependent kinase progression has been proven to block cell cycle progression, induce apoptotic cell death, promote differentiation, inhibit angiogenesis, and modulate transcription (9). Application of Staurosporine to mammary epithelium has shown the apoptotic effects of this reagent (9). A cell culture of similar origin should thus exhibit the same reaction. In this study, Thapsigargin and Staurosporine were added in a dose dependent manner to MCF-10-2A, a non-tumorigenic transformed cell line. The effects of each reagent on cell proliferation, tissue formation, and senescence (either necrotic or apoptotic) was examined via microscopic observations. These results were used to make inferences as to the susceptibility of this particular tissue formation to each reagent. Methods and Materials Media for Cell Line: Three media types were used to grow MCF-10-2A, non-tumorigenic, transformed epithelial cells from a fibrocystic disease in a mammary gland (2). Basal media was comprised of a 2% serum of fetal origin, essential components to support growth (e.g., glucose, essential Amino Acids, vitamins, isosmotic H2O via small ions), and appropriate buffers. In addition to Basal constituents, Growth media contained epidermal growth factor (EGF) and insulin, whereas Differential Media added the hormones insulin, cortisol and prolactin to Basal media. Well Preparation: The comparison of growth patterns in different media types was done using four variable well conditions. Each of four types contained an extracellular matrix component, either collagen IV, fibronectin, laminin or control blank (i.e., plastic) which was applied prior to seeding each well. Preparing Cell Cultures: In order to seed the wells with an equal number of cells, the prepared culture was split from a single flask and quantified using a hemocytometer. Cells were washed with 10 mL BSS. 4 mL BSS-trypsin was added and incubated for 5-10 minutes until cells were spherical and fully non-adherent. Trypsinized cells were added to 2 mL of F12-Growth media. Any cells remaining in the flask were removed by washing with 6 mL of F12-Growth medium, which was also added to the culture. Contents were centrifuged and the pellet was resuspended in 1mL F12-Basal medium and 3 mL regular Basal medium. 0.2 mL of resuspension was mixed with 0.2 mL of 0.4% Trypan Blue. The mixture was applied to a hemocytometer in order to determine cell density and amount needed to achieve desired well seeding density of 1.0 x 10^5 cells/well. Feeding Cells: All media types were aspirated and replaced with fresh media of same composition after a period of 24 hours. Cells were subsequently fed in an identical manner every 48 hours, and growth patterns were observed and recorded via phase contrast microscopy. Addition of Apoptotic Reagents: After 7 days of incubation at 32°C, 5% CO2, media was replaced with either basal media or four sequential dilutions (5.0 uM, 1.0 uM, 0.5 uM, 0.1 uM) of Staurosporine (in what had been differential media) and Thapsigargin(in what had been growth media). Observations were made after 5 and 18 hours. Results Cell Culture in Basal, Differential and Growth Medium After 24 hours (t=24), the first media change was performed, and cells were observed. There were no clear distinctions in growth patterns between media type. Cells were either fanshaped and independent from one another or cuboidal and arranged in a mosaic pattern. (Table 1, t=24) The mosaic pattern appears to be the initiation of tissue formation, with the cells in this arrangement appearing to be of similar size, shape and thickness. Neither cell morphology was more prevalent in each field of vision; independent cells were evenly dispersed between clumps of cells, which appear to be initiating tissue formation. Cells covered just over half the area of each well, indicating that they were 50-60% confluent. At 72 hours (t=72), cells were 90-100 % confluent in all experimental media. There were no distinctions in growth patterns among any of the given substratum components or various media types: basal, growth, differential. Cells were actively growing and dividing as indicated (Table 1, t=72) through the appearance and actions of now visible nuclei. Some cells appeared to be multi-nucleated, suggesting cell division was about to occur. Nuclei were one of the only visible internal cell components, and they took up the majority of the space. This phenomenon was apparent in all cells, which, regardless of media and substratum components in the well, appeared homologous with respect to size and shape. All cells existed in a single tissue layer with no visible overlaps or rounding (FIGURE 1). However, at a certain point in the periphery, there was a clear demarcation between cells that were part of the monolayer versus those that remained independent. These single cells still exhibited a fan-like conformation. Just prior to the addition of apoptotic reagents (t=7 days), cell density, uniform throughout, was slightly greater than previously seen at 72 hours. The cells were now approximately 100-110% confluent, indicating overgrowth. The first signs of cell death due to necrosis were visible, particularly in basal media wells (FIGURE 2). These cells were round and larger than those in the flat epithelium-like tissue, which underlies this cell type. The internal components of these cells were condensing into bright spots while the cytoplasm was much clearer in comparison to viable cells. Tissue formation occurring in growth medium exhibited a brighter outline around cells. In addition, the basal, plastic monolayer was comprised of cells that were visibly rounder than cells in other wells although they appeared to be just as numerous. Addition of Apoptotic Reagents: Staurosporine and Thapsigargin Observations five hours (t = A5) after the addition of Apoptotic reagents (Staurosporine to cells previously in Differential Media, and Thapsigargin to those previously in Growth), few changes were visible. Basal cells were indistinguishable from their appearance five hours ago. Overall, all cells subject to apoptotic reagent addition appeared slightly grainy, but the effect was minimal. The only visible death was a few necrotic cells in all media types. These cells were floating above the substratum, with internal components condensing and overall clearing of the cytoplasm without any change in cell size. All basal wells had a similar appearance, and the biggest change after 18 hours (t = A18) was a slight overall rounding of the cells (edges and more three dimensionally, they were not as flat as they were prior to addition of reagents). The most concentrated addition of Staurosporine, 5.0uM, resulted in widespread cell death. Most cells shrunk in size and appeared shriveled (FIGURE 3). For the first time prior to reaching confluency, the substratum was visible. Some cells clumped together, while others were separate and equidistant from all other cells. The lower concentration of 1.0uM Staurosporine had no visible effects on tissue growth as compared to the nature of the cells prior to the addition of the apoptotic reagent. Subsequent decreased concentrations exhibited the same phenomenon. Thapsigargin in its least diluted state, 5.0uM, also had a much smaller effect on this culture (FIGURE 4). Cells appeared viable, but were no longer in a flat, uniform monolayer. All cells were rounded and their morphology was similar to that of independent cells prior to reaching 100% confluency. In addition, there was also an increase in overall cell brightness. Discussion One of the hallmarks of the non-tumorigenic mammary epithelial cell line observed was similar growth trends and cell morphology across a variety of media and substratum conditions. The similarity in the time it took for each test well to reach various levels of confluency (TABLE 1) implies that components of the media and substratum had minimal effects on cell proliferation. This phenomenon was not observed in tumorigenic cell lines (data from Britton Keeshan and Andrew Fanous), which showed reactions specific to single experimental conditions (e.g., Growth medium on a laminin substratum). In all cases studied, transformed cells exhibited abnormal growth on the laminin substratum. Abnormal growth is characterized by non-uniform cell shape, the formation of organoids, and a lower level of cell adherence to substratum. (Table: Britton Keeshan and Andrew Fanous, Differential/Laminin: 96 hrs)(FIGURE 5) The failure to see distinctions in growth rates between media types in all cell lines was unexpected. Previous studies indicate that growth supplements found in Growth and Differential media, such as insulin, growth factors and other hormones, influence the rate of tissue development (3, 4). The similarities seen in cell growth, conformation and morphology in different media types may be the result of insufficient type and length of observation. Cells may have exhibited differences that were not readily observed using microscopy, or they may simply have required a longer period of time to differentiate. One other explanation is that perhaps the basal medium contained adequate supplements to allow for optimal cell growth and the additional supplements in growth and differential media were unnecessary. In addition to cell growth, observations also focused on cell shape. MCF-10-2A cells exhibited one of two main morphologies, either a slightly rounded, fan shape or a flat, seemingly adherent, cuboidal cell. These conformations were characteristic of independent or confluent cells in contact with one another, respectively. The change from fan-shaped to cuboidal is indicative of either an internal or external change in cellular activity, or possibly a combination of the two factors. These changes might include the secretion of different extra-cellular matrix and basement membrane components or the recruitment of cytosolic and cytoskeletal proteins (6). These changes affect cell to cell interactions, such as binding, signaling, or secretion. Thus, these interactions are now a likely explanation for the phenomenon of changing cell shape, as cuboidal cells exist in a mosaic pattern, which would enable cross talk between cells. Tissue formation generated from MCF-10-2A cells was comprised of cells that were approximately the same size, shape, thickness, and located in the same plane of growth. The only exception was seen in a single well of control (Basal) media, which ironically lacked any test substratum. These cells were slightly rounder and appeared less adherent. The similarity in cell density indicates that this observation is most likely a result of insufficient substratum components to maintain the formation of a MCF-10-2A monolayer. Weaver et. al. demonstrated that a mouse mammary epithelial cell line was unable to form a functional basement membrane when cultured on plastic substratum, and thus adherence and a successful cell culture was not achieved (6). Overall, tissue formation was not greatly influenced by media or substratum type in nontumorogenic transformed cells. Similarly, cell morphology did not seem to depend significantly on either of those conditions as well. Rather, cell-cell interaction appeared to be of greatest importance in eliciting cell shape. Senescence is an event that contributes to tissue differentiation. After one week of cell culture, cell death was not widespread, and as a result, all tissues appeared the same. We assume that similar conformation was indicative of similar function, and thus the result of apoptotic induction can be attributed solely to the reagents added and not cellular constraints. Cell death can occur by two morphologically and functionally distinct methods, Necrosis and Apoptosis. The only cell death seen prior during the first week of cell culture was an insignificant amount of necrotic cells visible in all conditions. Phase contrast microscopy indicated that cells located above the cultured monolayer of non-tumorigenic epithelial cells exhibited characteristics of necrosis, including cell swelling which is a result of membrane disruption. Large bright spots inside the cell are indicative of the condensation of internal components, another hallmark of necrosis (Figure 5)(10). The lack of programmed cell death, or apoptosis, indicates that this cell culture is not actively participating in this activity during this phase of growth. After seven days of observations, though cell cultures were 100% confluent, cellular constituents of each monolayer became smaller at each time interval, indicating continued growth rather than death or elimination of cells. Lack of apoptosis in the first phase of cell culture may be attributed to either internal or external factors. This cell line may not utilize this mechanism or it may not yet have reached a stage in its life cycle that exhibits apoptosis. The MCF-10-2A cell line exhibits the ability to undergo apoptosis, as effects were seen in a dose dependent manner using apoptotic reagents Thapsigargin and Staurosporine. As expected, cultures treated with Staurosporine show signs indicative of apoptosis, including reduced size and apparent chromatin condensation, indicated by bright spots in each apoptotic cell (Figure 6)(1, chris source). The effect was greatest at a concentration of 5.0uM, and concentrations less than 1.0uM indicated no signs of apoptosis. This is most likely a result of decrease reagent concentration and not attributed to cell culture characteristics, as growth was similar in all wells regardless of substratum components prior to addition of Stauosporine, and control wells containing basal medium continued to indicate identical growth patterns in all substratum types. The reaction to the addition of Thapsigargin is not easily identified as apoptotic. The only effect was seen in a concentration of 5.0uM, and cells appear slightly smaller, but rounded and bright along the edges (Figure 7), not commonly seen in apoptotic cells. The physical changes in the cell line might be attributed to a reaction between Thapsigargin and the substratum or media components (collagen IV and differential, respectively), which altered certain pathways. A lack of previous experimentation on the effect of Thapsigargin on mammary epithelial cells makes it difficult to attribute any result to a specific causal agent. In summary, there were no significant variations observed in either tissue formation or reactions to external changes using this non-tumorogenic cell line. The transformed nature of the cells may inhibit reactions which normally occur in the host from which they are derived from (1). Tissue culture studies are effective for visualization of reaction of cells that are in contact with one another and various external components. The minimal data obtained from these experiments is indicative of one of the main caveats of cell culture, mainly that they are not the original cells but cloned copies which have undergone numerous passages. This in addition to other factors may contribute to the need to improve current methods. Future Work Cell Culture 1. Disrupt intercellular signaling and see what effect that has on cell morphology. Use antibodies for integrins, which are trans-membrane receptors that transmit signals from the extracellular matrix, to impede cell-cell signaling (6). 2. Quantify and differentiate between apoptosis and necrosis. Propidium iodide can be used to stain for necrosis. Quantitative measurements of apoptosis and necrosis can be taken using a flow cytometer. Apoptosis 1. Allow tissue formation to proliferate for a longer period of time depending upon resulting growth and alterations in intracellular function under the same conditions applied to the experiments outlined in this paper. Record results at sufficient intervals. Using the information from current experiments, record initial changes during the first 24 hrs and every 72 hrs thereafter. 2. Upon observation of significant instances of cell death, quantify the method of senescence (apoptotic vs. necrotic) in proportion to one another by using different stains such as annexin and propidium iodide and a cytometer. 3. Apply Staurosporine and Thapsigargin in a dose dependent manner as outlined in the Methods section. Utilize the same quantification techniques for each method of cell death as done on untreated cultures. Use cells grown on the same substratum to eliminate this factor as a confounding variable. Compare the proportion of necrotic and apoptotic cells to those found in uninduced cultures. Conclude which reagent is best to mimic normal cell conditions and use this reagent in subsequent studies with this cell line. Figures FIGURE 1: MCF-10-2A cells in growth medium with laminin substratum, prior to addition of apoptotic reagents (t = 72 hrs). Observations such as homologous cuboidal shaped cells arranged in a mosaic pattern, uniform cell size, visible nuclei, and a monolayer of cells were characteristic of all cultures prior to the addition of apoptotic reagents. FIGURE 2: MCF-10-2A cells in Basal medium with plastic substratum immediately prior to the addition of apoptotic reagents (t = 7 days). Initial signs of cell death due to necrosis are visible. Cells are slightly rounder and larger than those that composed the flat epithelium tissue observed previously. Condensed internal cellular components are visible as bright spots in the cytoplasm. FIGURE 3: MCF-10-2A cells after the addition of Staurosporine at a final concentration of 5.0 uM. Widespread cell death is visible. Cells no longer completely cover the substratum in an uninterrupted single-layer tissue. Instead areas of substratum without any cells are now visible. Cells that are left appear shriveled and shrunken in size. FIGURE 4: MCF-10-2A cells after the addition of Thapsigargin at a final concentration of 5.0 uM. Thapsigargin shows less effect as an apoptotic reagent than does Staurosporine. Cells still appear viable; although, they are no longer as flat and arranged in a uniform monolayer. Instead they are more three-dimensional and rounded along the edges. An increase in brightness around cell edges is also noticeable. Figure 5:(from Britton Keeshan and Andrew Fanous): T47D, a tumorigenic cell line, grown in Differential medium with laminin substratum (t = 96 hrs). Transformed cells exhibit abnormal growth on the laminin substratum. Evidence can be seen of non-uniform cell shape, the formation of organoids, and a lower level of cell adherence to substratum. Non-adherent cells above the monolayer exhibit characteristics of necrotic cells: cell rounding and condensation of internal components (10). Figure 6: MCF-10-2A cell culture treated with Staurosporine (5.0uM). Reduced cell size and chromatin condensation, reflected by bright spots in the cells, are indicative of apoptosis. Also there are now large spaces without any cell growth. Figure 7: MCF-10-2A cells treated with Thapsigargin (5.0uM). While cells appear smaller, they also seem more rounded and brighter around the edges, which are qualities not often associated with apoptosis. No cell-free areas are visible. Overall, the apoptotic effects of Thapsigargin are less dramatic compared to those of Staurosporine. Tables TABLE 1 MCF-10-2A (Alicia and Sophia) Overall 0 24 Trends no (no pictures) obs -majority of cells are erv in fan-like ati conformation ons -50-60% confluency evenly distributed throughout wells Basal Growth Differential C -cells forming tissues show cuboidal shape in mosaic pattern -single, independent cells are fan-shaped -both cell types are equally represented L F P Same Same Same 72 -90-100 % confluency -tissue formation concentrated in center of well -periphery is greatly reduced -minimal independent cells (no pictures) -cell conformation and shape are uniform -rate of growth has slowed -well-defined nucleus that takes up large proportion of each cell -adherence and cell thickness are also consistent throughout tissue formation -same -same -same C Same - same L Same -same F P C Same Same Same - same -same -same Pre-apoptosis -100% confluency -first signs of necrosis -uniform single layer of cells -all cells flat, no signs of rounding or dome-like structures Apop 5 hrs BASAL-basal DIFFERENTIAL – Staurosporine GROWTH Thapsigarogin Apop 18 hrs BASAL-basal DIFFERENTIAL – Staurosporine GROWTH Thapsigarogin -cells are slightly less uniform in relation to one another -no changes in cell conditions for all wells from observations prior to apoptotic reagent addition -no distinction between all wells with basal media -all cells appear more rounded, still flat and confluent -same as Basal C - same as Basal C - edges of cells appear more rounded in the monolayer (Figure 1) -no significant differences as a result of different substratum -cell edges are brighter in all growth conditions in comparison to other media types (Figure 2) -same as Growth C -same -same -same -same -same -same -cell edges are less defined and cell interior appears grainy -no change in confluency -nucleus still visible but not as distinct (5.0 uM) -cells appear elongated (similar to confirmation prior to reaching confluency) and much brighter than any prior observations (whole cell, not just edges) (Figure 4) -same -same as Growth C -same as Growth C -most even and uniform cell growth as compared to all other conditions (time of incubation -same -same -same (1.0 uM) -tissue formation is identical to conditions seen prior to addition of reagents -some web-like cells visible in all wells -same -same (5.0 uM) -widespread apoptosis -cells are much smaller, shriveled appearance, internal components are no longer distinguishable and media) L Same -same -same as Differential C -same -first time cells are rounded and multiple layers are apparent (Figure 3) (1.0 uM) -tissue formation is identical to conditions seen prior to addition of reagents F Same -same -same -same P Same -same -same as Differential C -same as Differential C -same -same References 1. Watters, C.D. 2005. An Introduction to Cell Culture. Practical Cell Biology (BI360). P 15 2. ATCC catalog, accessed 2/21/05 3. Butler, M. and Dawson, M. 1992. Cell Culture. LabFax. Academic Press (New York) 247 pp. 4. Freshney, R.I. 2000. Culture of Animal Cells. 4th ed. Willey-Liss (New York) 577pp. 5. Cross, P.C. and Mercer, K.L. 1993. Cell and Tissue Ultrastructure. W.H. Freeman 420 pp. 6. Weaver, V.M. and Bissell, M.J. 1999. Functional Culture Models to Study Mechanisms Governing Apoptosis in Normal and Malignant Mammary Epithelial Cells. J. Mamm. Gland Biol. and Neoplasia. 4 (2: 193-200). 7. Biocoat catalog, 1994. Innovative Products for Cell Science Research. Becton Dickinson Labware 8. Saleh, H., Schlatter, E., Lang, D., Pauels, H.G, and Heidenreich, S. 2000. Regulation of mesangial cell apoptosis and proliferation by intracellular Ca2+ signals. Kidney Intl. 58: 1876-1884. 9. Chen, X., Lowe, M., Herliczek, T., Hall, M.J., Danes, C., Lawrence, D.A., and Keyomarsi, K. 2000. Protection of Normal Proliferating Cells Against Chemotherapy by Staurosporine-Mediated, Selective, and Reversible G1 Arrest. JNCI. 92(24: 1999-2008). 10. Los, M., Mozoluk, M., Ferrari,D., Stepczynska, A., Stroh,C., Renz, A., Herceg, Z., Wang, Z., and Klaus Schulze-Osthoff. 2002. Activation and Caspase-mediated Inhibition of PARP: A Molecular Switch between Fibroblast Necrosis and Apoptosis in Death Receptor Signaling. Mol Biol Cell. 13(3): 978–988.