strengthening mechanisms
advertisement

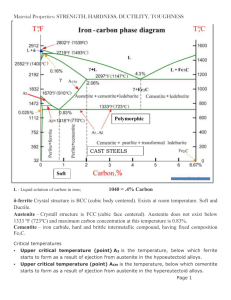
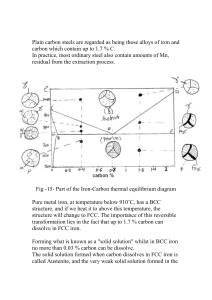
13.3 STRENGTHENING MECHANISMS The use of steel as a structural material is based, in large part, on its strength and ductility. Both of these properties are related to the movement of dislocations through the crystal lattice of the material (see Chapters 2 and 5). Measurable plastic deformation, or yielding, requires that there be an applied stress and that two other processes occur consecutively: (1) Dislocations must be generated at some type of dislocation source, and (2) these dislocations must move appreciable distances through the crystal. The mechanism, either dislocation generation or dislocation movement, that requires the higher stress controls the yielding behavior, and thus the mechanical properties, of the steel. Strengthening of a metal to increase its resistance to yielding or plastic deformation can be obtained by changes in microstructure that impede the motion of dislocations. Structural steels can be strengthened through mechanisms such as (1) the introduction of interstitial and substitutional atoms (alloying), (2) the generation and concentration of dislocations (work or strain hardening), and (3) the formation of additional grain boundaries (heat treatment). Alloying and heat treatment processes combine synergistically to produce a tremendous variety of microstructures, and hence properties, in structural steels. 13.3.1 Alloying Alloying elements affect the microstructure and, therefore, the properties of steel through the following mechanisms: 1. Formation of a solid solution with iron, resulting in solid-solution strengthening and increased corrosion resistance (carbon, chromium, manganese, nickel, copper, and silicon). 2. Formation of a carbide (i.e., a binary compound of carbon and a more electropositive element); such carbides impart additional hardness and elevated temperature strength (titanium, vanadium, and molybdenum). 3. Formation of an undissolved, second phase which promotes machineability (lead, sulfur, and phosphorus). In addition, all alloying elements promote hardenability (a measure of the capability of a steel to harden throughout the cross section of a bar or component during 2 heat treatment) and facilitate the nonequilibrium transformation of austenite to microstructural products other than pearlite. This may be beneficial in high strength steels, but it can cause problems in welding. Elements which readily form oxides (aluminum, silicon, calcium, and manganese) are also added to steel to remove dissolved gaseous oxygen. Otherwise, this dissolved oxygen will come out of solution on cooling to form small bubbles in the solid steel. The chemical composition and mechanical properties of deoxidized or 'killed' steels are more uniform than those of other types. The raw materials used to make steel contain other elements which are undesirable but appear in the resulting products. The amounts of these residual elements are usually held to acceptable limits by careful steel-making practice, and they are not generally reported. However, certain of these elements (sulfur and phosphorus, for example) negatively affect the mechanical properties of steel. Hence restrictions are placed on the amounts of such elements allowed in most grades of steel, and these amounts are normally reported in the analysis of both plain carbon and low alloy steels. The strengthening and hardening that result from a given alloying element in solid solution depend on the difference between its atom size and electron structure and those of the solvent metal. In dilute solid solutions, the amount of strengthening is roughly proportional to the concentration of the alloying element. If more than one solute element is present, the total strengthening is approximately the sum of the characteristic effects of each element taken alone. Solute atoms that are simply distributed at random in the solvent impede the motion of dislocations to a limited extent, and therefore impart only modest strengthening. However, if the solute atoms collect preferentially around existing dislocations, these dislocations are effectively locked and the force required to move them may be increased greatly. Both substitutions solid-solution atoms, which are either appreciably larger or smaller than the matrix atoms, and interstitial atoms can lock dislocations. Carbon and nitrogen are interstitial alloys that exert a pronounced strengthening effect on iron in this way. The properties of the steel for a given alloying composition can be further modified by changing the microstructure by a variety of mechanical treatments and heat treatments which involve solid-state transformations. These are discussed in the following sections. 13.3.2 Work (Strain) Hardening All pure metals and alloys can be strengthened by cold working, or plastic deformation below the recrystallization temperature. Recrystallization is a process in which new strain-free grains are nucleated and grow. It occurs when the metal or alloy is heated above a certain temperature-the recrystallization temperature. Because the recrystallization temperature is several hundred degrees above room temperature for steels, shaping or working at and near ambient temperatures is cold-working. In this process, excessive concentrations of dislocations are generated during initial straining (working), and a complex network of dislocations is produced. This network makes further dislocation motion extremely difficult because of mutual interference. The resistance to further deformation increases with increasing amounts of deformation, a phenomenon called strain-hardening, or workhardening. Thus, easy slip in the crystal lattice is reduced and the alloy is strengthened. However, the ductility and toughness of the metal or alloy decrease when it is work- or strain-hardened (Fig. 13.6). Materials strengthened appreciably in this way, therefore, tend to have low ductility. Furthermore, materials strengthened by work hardening cannot be joined by welding without softening (annealing) the material in the vicinity of the weld. For these reasons, this method of strengthening finds little application in structural steels. 3 Figure 13.6 Change in mechanical properties associated with work- or strain-hardening. 13.3.3 HeatTreatment Structural or mild steels are usually employed in the 'as-rolled' or non-heat-treated condition. However, to obtain certain properties, it may be necessary to alter the microstructure of the material from that found in this condition by subjecting the steel to various heat treatments. All of these processes are directed toward producing the mixture of ferrite (a) and cementite (Fe3C) that gives the proper combination of properties. In general, a heat treatment may be defined as an operation or series of operations involving the heating and cooling of a metal or alloy in the solid state to produce desirable conditions or properties. Of primary interest, insofar as structural steels are concerned, are changes which occur on cooling of the material from some point above one of the critical temperatures (i.e., A1 or A3; Fig. 13.4). The temperature to which the material is heated before cooling is begun determines the phases which are present at the end of the cooling process, as well as at the beginning, whereas the form of these phases (the microstructure of the heat-treated steel) is determined by the cooling temperatures and rates. Isothermal Heat Treatments The solid-state eutectoid reaction resulting in the formation of ferrite () and cementite (Fe3C) from austenite () is rather slow, and the steel may cool below the equilibrium eutectoid temperature (A1) before this transformation is complete. If the transformation occurs at temperatures below A1, a nonequilibrium structure will form, and strengthening will probably result. One convenient method of describing the nonequilibrium transformation of austenite during cooling is the isothermal transformation diagram, referred to more commonly as the time-temperature-transformation diagram, or TTT diagram (Fig. 13.7(a)). This diagram shows the results of transforming austenite isothermally (i.e., at constant temperature) at temperatures below the upper critical temperature (the temperature at which the alloy under consideration is fully austenitic-A3 for hypoeutectoid steels). 4 Figure 13.7 (a) Isothermal transformation (TTT) diagram for a hypoeutectoid steel (A, austenite; F, ferrite; and C, iron carbide [Fe,C]): Transformation of austenite starts at curve a, and is complete at curve c; (b) photomicrograph showing bainitic structure in a hypoeutectoid steel (X750); (c) photomicrograph showing martensitic structure in tempered hypoeutectoid steel (X750) (P. A. Thornton and V. J. Colangelo, Fundamentals of Engineering Materials, Prentice-Hall, Inc., 1985. Photomicrographs courtesy of Theresa Brassard). 5 In Fig. 13.7(a), the shaded portion indicates the region where transformation is occurring as a function of time for a particular (constant) temperature. For example, at a temperature above 550C (the nose of the TTT curve), transformation of the austenite to ferrite begins at curve a and continues isothermally until curve b. Austenite then begins to transform to pearlite and continues this reaction until curve c is reached. At c the austenite is completely transformed and a microstructure of ferrite and pearlite results. At temperatures below the nose of the TT T curve, transformation of austenite produces a structure in which the ferrite and iron carbide are not lamellar, as in pearlite. This transformation product, called bainite, exhibits a fine, feathery or acicular (needlelike) structure (Fig. 13.7(b)). Depending on the actual transformation temperature, this nonequilibrium transformation product may have a higher strength and hardness than pearlitic structures while maintaining usable ductility and toughness. Transformation of austenite at temperatures lower than those in the bainite range occurs as a function of temperature only; transformation is independent of time. This process involves a diffusionless transformation which starts at a temperature labeled MS, and produces a nonequilibrium or transition product called martensite, which has a fine grain size and is very hard and brittle (Fig. 13.7(c)). However, this phase is not stable and, given an opportunity, will proceed to form ferrite and iron carbide; as a result, we do not see martensite on the Fe-Fe3C phase diagram. Normally, steels containing martensite are further heat treated (tempered) to produce more desirable microstructures and properties. Continuous Cooling Heat Treatments In practice, structural steels are strengthened through microstructural transformations which take place under continuous cooling conditions rather than isothermal conditions. In continuous cooling heat treatments, the material is cooled from above the A3 temperature to room temperature continuously; the transformation from austenite to pearlite and/or martensite therefore occurs over a range of temperatures instead of at a single temperature, resulting in a more complex microstructure. The products formed by continuous cooling procedures can be predicted using a continuous cooling diagram (Fig. 13.8), which differs from the isothermal transformation diagram in that the beginning and the end of the transformation are generally shifted to lower temperatures and longer times. Different rates of cooling, and hence different microstructures, are achieved through the use of different cooling media, as shown in Fig. 13.8. Essentially, as the cooling rate is increased, the hardness and the strength of the steel increase. Rapidly cooling, or quenching, the austenite at a rate equal to or greater than the critical cooling rate (Fig. 13.8) will bypass the knee of the TT T curve and result in the formation of martensite. However, martensite is too hard and brittle for most purposes, and it is usually transformed to the equilibrium phases ( and Fe3C) using a lowtemperature heat treatment called tempering. The resulting microstructure is not lamellar, like that of pearlite, but contains many dispersed carbide particles. Tempering causes the strength and hardness of the martensite to decrease, while the ductility and impact properties are improved. By selecting the appropriate tempering temperature, a wide range of properties can be obtained. Heat treatment processes involving slower cooling rates are primarily carried out to reduce or eliminate the undesirable effects of prior treatment of the steel. Two processes are in common use: normalizing and annealing (Fig. 13.9). In the 6 Figure 13.8 Variation of microstructure as a function of cooling rate for an eutectoid steel (after R. E. Reed-Hill, Physical Metallurgy Principles, D. Van Nostrand Co. Inc., 1964). Figure 13.9 Schematic representation of heat treatment processes for a hypoeutectoid steel. normalizing process, the material is heated to a temperature above the austenitizing temperature and, after a suitable holding time, cooled in still air, forming a fine pearlite microstructure. The principal effect of normalizing is that grain refinement occurs (i.e., grain size decreases), resulting in improved ductility and toughness. Furthermore, since recrystallization of the material occurs in this process, variations in the mechanical properties, caused by differential cooling rates following rolling cycles, will he reduced or eliminated. Thus, these properties will be more uniform, within a piece, and from piece to piece, than is the case with as-rolled steel. 7 In the full annealing process, the material is heated to a temperature above the austenitizing temperature and then slowly cooled in the furnace with the following effects: (1) The material is softened and its ductility is improved; (2) internal stresses induced, for example, by strain or work hardening, are relieved; and (3) recrystallization occurs. However, a partial annealing process, called process annealing or stress relieving, is more commonly used than full annealing: The steel is heated to a temperature below, but close to, the austenitizing temperature, followed by any desired rate of cooling. Process annealing is primarily carried out to relieve internal stresses resulting from work- or strain-hardening, but recrystallization may also occur.