Document name:
advertisement

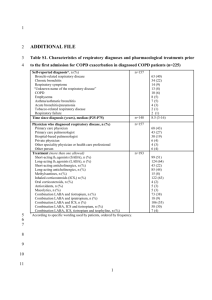
Document name: Guidelines for the recommendation of inhaled medication changes for COPD Document type: Guideline Staff group to whom it applies: Registered Nurses whether they are prescribers or not who are recommending changes to inhaled therapy for COPD Distribution: The whole of the Trust How to access: Intranet and internet / ward folder Issue date: June 2015 Next review: June 2017 Approved by: Executive Management Team Developed by: Practice Governance Coach Director leads: Director of Nursing, Clinical Governance and Safety Contact for advice: Kath Hemming Practice Governance coach Julie Booker Lead Nurse for COPD Sarah Hudson Lead pharmacist Barnsley BDU Contents Content Introduction and Purpose Page Number 3 Scope 3 Definitions 3 Duties and Responsibilities 3 Process 4 Training 4 Appendices 1. Algorithm for inhaled therapies 5 2. Notification of Change of Medication 8 2 Introduction and Purpose South West Yorkshire Partnership Foundation Trust employs a number of Registered Nurses who work in specialist or clinically advanced roles who are not registered with the Nursing and Midwifery Council, (NMC) as a nurse independent prescriber or a nurse supplementary prescriber. An example of this is COPD Specialist Nurse and Assistant Community Matrons. These nurses will review and assess on a regular basis patients with a diagnosis of Chronic Obstructive Pulmonary Disease (COPD). Their assessment, based on best available evidence and in partnership with the patient, may indicate that a change in inhaled therapy is recommended. This guideline has been developed to ensure that Registered Nurses working with patients with a diagnosis of COPD, who do not hold a nurse independent/supplementary prescribing qualification and make recommendations to a GP or a nurse independent prescriber based on their clinical assessment, do so within a framework of best available evidence from NICE and locally agreed inhaled therapy management algorithm for COPD shown in Appendix 1. It is recommended as best practice that registered nurses holding an independent prescribing qualification also prescribe inhaled therapy in line with this algorithm. Scope This guideline applies to all Registered Nurses who do not have an independent or supplementary prescribing qualification, working with patients with a diagnosis of COPD who have completed a COPD competency assessment framework. This guideline only applies to inhaled therapies and must be read in conjunction with the Trust Medicines Code. Definitions Chronic Obstructive Pulmonary Disease is characterised by airflow obstruction that is not fully reversible. The airflow obstruction does not change markedly over several months and is usually progressive in the long term Nurse Independent Prescriber - Specially trained registered nurses allowed to prescribe any licensed and unlicensed drugs within their clinical competence. Nurse Supplementary Prescriber - voluntary prescribing partnership between a doctor (independent prescriber), a registered nurse (supplementary prescriber) and the patient where the supplementary nurse prescriber has the ability to prescribe any drug listed in a patient-specific clinical management plan once the patient has been diagnosed by a doctor FEV1 – (Forced Expiratory Volume in 1 second) – The amount of air that can be forcibly expired in the first second of a forced exhalation, it is measured through spirometry. Duties and Responsibilities The COPD Lead Nurse is responsible for ensuring appropriate training and supervision is available for Registered Nurses following this guideline Registered Nurses reviewing and making recommendations for changes to inhaled therapy regimes are responsible for ensuring their competencies for the management of patients with COPD are up to date with current evidence based treatments for the management of COPD. Registered Nurses reviewing and recommending changes to inhaled therapy regimes are responsible for reporting any untoward incident in relation to this guidance in line with the Trust’s Policy. 3 Process Following full assessment and in partnership with the patient, recommendation for changes to inhaled therapy for the management of COPD will be in line with the following algorithms, (Appendix 1) Algorithm for inhaled therapies in the management of COPD Algorithm for inhaled therapies in the management of COPD for patients with FEV1>50% Algorithm for inhaled therapies in the management of COPD for patients with FEV1 < 50% Options for changes to inhaled therapies and recommendations will be discussed and agreed with the patient. The patient must be made aware that further discussion will be held with their GP or appropriate nurse independent prescriber. Recommendations made to the GP or nurse independent prescriber, where possible, must be made for the inhaled therapy drug group, e.g. long acting beta agonist. However, there may be times when a particular drug is recommended, for example, where a patient has been assessed as suitable for a particular inhaler device, and this device is only available for a particular drug. Any recommendations for changes in inhaled therapy must have a clear rationale, i.e. findings of assessment, any changes in observations that is discussed or put in writing to the GP or independent prescriber and recorded clearly within the patient’s clinical record. A template letter for recording the rationale and recommendations to changes in inhaled therapy is available in Appendix 2. The patient must be informed of the outcome of the recommendation to the GP/ independent prescriber and be made aware of how they will receive supplies for any changes to the inhaled therapy. For situations where the patient requires immediate treatment for an acute exacerbation, the registered nurse can administer the patient’s own salbutamol via the usual method of delivery in the following way: For moderate exacerbations, give 2 – 10 puffs of salbutamol each inhaled separately and repeated after 10- 20 minutes as necessary. If there is a poor clinical response after 30 minutes, arrange immediate transfer to hospital. For acute exacerbations, start treatment as described above and arrange immediate transfer to hospital. Training All Registered Nurses using this guideline must be trained and assessed as competent in the management of COPD, using an appropriate framework and attend regular updates as required. All new staff meeting the above criteria who work with patients with a diagnosis of COPD will be made aware of these guidelines at local induction. References 1. NICE Clinical Guideline 101 (2010) Chronic Obstructive Pulmonary Disease 2. Algorithm for Inhaled Therapies in the Management of COPD, (2014) Adapted from Barnsley Guidance on the Management of COPD 4 Appendix 1 Adapted from Barnsley guidance on the management of COPD and based on NICE Clinical Guideline 101 – Chronic Obstructive Pulmonary Disease June 2010 Algorithm for inhaled therapies in the management of COPD For patients with an FEV1<50% Breathlessness or Exercise limitation Exacerbations or persistent breathlessness Use SABA (Salbutamol or Terbutaline) or SAMA (Ipratropium) 2 puffs QDS PRN Per device: Salbutamol inhaler £1.50, Salbutamol Easyhaler £3.31, Salbutamol Easibreathe inhaler £6.30, Salbutamol Accuhaler £4.85, Terbutaline turbohaler £6.92, Ipratropium inhaler £5.56 LABA+ICS in combination inhaler Fostair (100 micrograms extra-fine beclometasone + 6 mcg formoterol) 2 puffs BD £29.32 Or Symbicort 400/12 Turbohaler (400mcg budesonide + 12 mcg formoterol) 1 puff BD £38.00 Or Seretide 500 Accuhaler (500mcg fluticasone + 50 mcg salmeterol) 1 puff BD £40.92 LAMA (in place of SAMA) st 1 line Tiotropium 18mcg OD Spiriva handihaler £34.87 with inhaler device, £33.50 refill pack nd 2 line Aclidinium 322mcg BD Eklira Genuair dry powder £28.60 Consider using either Salmeterol or Formoterol, plus Tiotropium or Aclidinium if inhaled steroid declined or not tolerated. Persistent Exacerbations or breathlessness LAMA+LABA+ICS Fostair (100 micrograms extra-fine beclometasone + 6 mcg formoterol) 2 puffs BD £29.32 Or Symbicort 400/12 Turbohaler (400mcg budesonide + 12 mcg formoterol) 1 puff BD £38.00 Or Seretide 500 Accuhaler (500mcg fluticasone + 50 mcg salmeterol) 1 puff BD £40.92 Plus Tiotropium 18mcg OD Spiriva handihaler £34.87 with inhaler device, £33.50 refill pack Or Aclidinium 322mcg BD Eklira Genuair dry powder £28.60 SABA – Short acting beta agonist SAMA – Short acting antimuscarinic LABA – Long acting beta Offer therapy Consider therapy 5 Adapted from Barnsley guidance on the management of COPD and based on NICE Clinical Guideline 101 – Chronic Obstructive Pulmonary Disease June 2010 Algorithm for inhaled therapies in the management of COPD For patients with an FEV1>50% Breathlessness or Exercise limitation Use SABA (Salbutamol or Terbutaline) or SAMA (Ipratropium) 2 puffs QDS PRN Per device: Salbutamol inhaler £1.50, Salbutamol Easyhaler £3.31, Salbutamol Easibreathe inhaler £6.30, Salbutamol Accuhaler £4.85, Terbutaline turbohaler £6.92, Ipratropium inhaler £5.56 LABA Exacerbations or persistent breathlessness LAMA (in place of SAMA) st 1 line Formoterol 12mcg BD nd 2 line Salmeterol 50mcg BD Reserve Indacaterol▼ for patients who require a once daily preparation Formoterol easyhaler £11.88 Formoterol mdi inhaler £18.04 Formoterol dry powder-Foradil £23.38, Formoterol turbohaler (Oxis) £24.80 Salmeterol Accuhaler and Evohaler £29.26; Indacaterol breezhaler £29.26 Persistent Exacerbations or breathlessness st 1 line Tiotropium 18mcg OD Spiriva handihaler £34.87 with inhaler device, £33.50 refill pack nd 2 line Aclidinium 322mcg BD Eklira Genuair dry powder £28.60 LABA+ICS in combination inhaler* Fostair (100 micrograms extra-fine beclometasone + 6 mcg formoterol) 2 puffs BD £29.32 *Please note: LABA+ICS combination inhalers are licensed for patients with an FEV1<50% (Fostair and Symbicort) or FEV1<60% (Seretide). However, can be considered in patients who remain symptomatic despite regular treatment with a long acting beta agonist. Or Symbicort 400/12 Turbohaler (400mcg budesonide + 12 mcg formoterol) 1 puff BD £38.00 Or Seretide 500 Accuhaler (500mcg fluticasone + 50 mcg salmeterol) 1 puff BD £40.92 Consider using either Salmeterol or Formoterol, plus Tiotropium or Aclidinium if inhaled steroid declined or not tolerated. SABA – Short acting beta agonist SAMA – Short acting antimuscarinic LABA – Long acting beta agonist Offer therapy LAMA – Long acting antimuscarinic Consider therapy ICS – Inhaled Corticosteroid LAMA+LABA+ICS Fostair (100 micrograms extra-fine beclometasone + 6 mcg formoterol) 2 puffs BD £29.32 Or Symbicort 400/12 Turbohaler (400mcg budesonide + 12 mcg formoterol) 1 puff BD £38.00 Or Seretide 500 Accuhaler (500mcg fluticasone + 50 mcg salmeterol) 1 puff BD £40.92 Plus Tiotropium 18mcg OD Spiriva handihaler £34.87 with inhaler device, £33.50 refill pack Or Aclidinium 322mcg BD Eklira Genuair dry powder £28.60 6 Algorithm for Inhaled therapies in the management of COPD (adapted from NICE Clinical Guideline 101 – Chronic Obstructive Pulmonary Disease (update) June 2010 Prices taken from Drug Tariff June 2014 (30 day costs unless otherwise stated). Use SABA (Salbutamol or Terbutaline) or SAMA (Ipratropium) 2 puffs QDS PRN Breathlessness or Exercise limitation Per device: Salbutamol inhaler £1.50, Salbutamol Easyhaler £3.31, Salbutamol Easibreathe inhaler £6.30, Salbutamol Accuhaler £4.85, Terbutaline turbohaler £6.92, Ipratropium inhaler £5.56 FEV1 > 50% FEV1 < 50% Exacerbations or persistent breathlessness LABA LAMA (in place of SAMA) 1st 1st line Formoterol 12mcg BD 2nd line Salmeterol 50mcg BD Reserve Indacaterol for patients who require a once daily preparation Formoterol easyhaler £11.88 Formoterol mdi inhaler £18.04 Formoterol dry powder-Foradil £23.38, Formoterol turbohaler (Oxis) £24.80 Salmeterol Accuhaler and Evohaler £29.26; Indacaterol breezhaler £29.26 Persistent Exacerbations or breathlessness Offer Therapy Consider therapy LABA+ICS in combination inhaler line Tiotropium 18mcg OD Spiriva handihaler £34.87 with inhaler device, £33.50 refill pack 2nd line Aclidinium 322mcg BD Eklira Genuair dry powder £28.60 LABA+ICS in combination inhaler Fostair 2 puffs BD Or Seretide 500 Accuhaler 1 puff BD Or Symbicort 400/12 Turbohaler 1 puff BD Consider using either Salmeterol or Formoterol, plus Tiotropium or Aclidinium if inhaled steroid declined or not tolerated. Fostair 2 puffs BD (Fostair mdi £29.32) Or Symbicort 400/12 Turbohaler 1 puff BD (Symbicort 400/12 turbohaler £38.00) Or Seretide 500 Accuhaler 1puff BD (Seretide 500 accuhaler £40.92) Consider using either Salmeterol or Formoterol, plus Tiotropium if inhaled steroid declined or not tolerated. LAMA+LABA+ICS Use Fostair, Seretide or Symbicort plus Tiotropium or Aclidinium Although not included within the NICE guidance, in patients with severe disease who remain breathless, at the discretion of the Respiratory Specialist, Seretide or Symbicort may be replaced by Indacaterol plus an inhaled corticosteroid plus Tiotropium. SABA=short acting bronchodilator SAMA=short acting antimuscarinic LABA=long acting bronchodilator LAMA=long acting antimuscarinic Guideline adapted June 2014 Dr H Mahdi, Respiratory Consultant and COPD Lead; Caron Applebee, Medicines Management Pharmacist, Barnsley CCG Appendix 2 SOUTH WEST YORKSHIRE PARTNERSHIP NHS FOUNDATION TRUST Community COPD Service Notification of Change of Medication Date: GP: Name: Patient’s Address: DOB: Unit No: Medication: Increased: Daily Dose: Medication: Decreased: Daily Dose: Rationale: Signature: ……………………………………….. Print Name: ………………………………… Respiratory Nurse Specialist / COPD Service Lead From: Community COPD Service Apollo Court Medical Centre High Street Dodworth Barnsley S75 3RF Tel: 01226 209889 Service Lead – Julie Booker Copy to: