Electrospray Ionization Method
advertisement
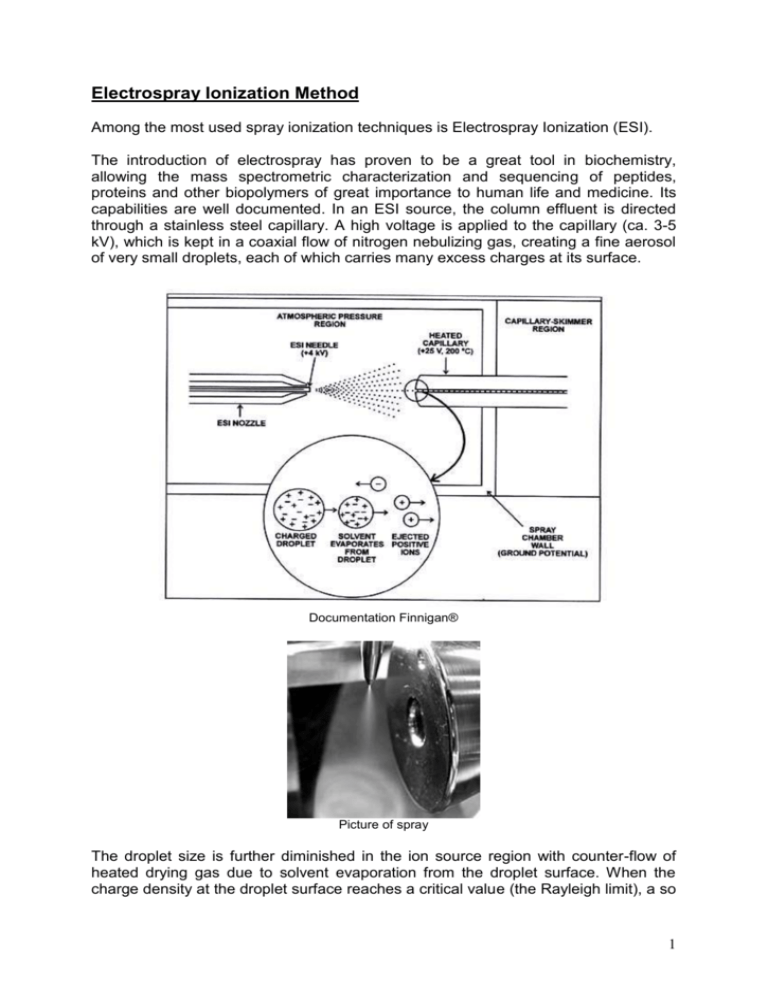
Electrospray Ionization Method Among the most used spray ionization techniques is Electrospray Ionization (ESI). The introduction of electrospray has proven to be a great tool in biochemistry, allowing the mass spectrometric characterization and sequencing of peptides, proteins and other biopolymers of great importance to human life and medicine. Its capabilities are well documented. In an ESI source, the column effluent is directed through a stainless steel capillary. A high voltage is applied to the capillary (ca. 3-5 kV), which is kept in a coaxial flow of nitrogen nebulizing gas, creating a fine aerosol of very small droplets, each of which carries many excess charges at its surface. Documentation Finnigan® Picture of spray The droplet size is further diminished in the ion source region with counter-flow of heated drying gas due to solvent evaporation from the droplet surface. When the charge density at the droplet surface reaches a critical value (the Rayleigh limit), a so 1 called Coulombic explosion occurs and several even smaller droplets are formed, each carrying some fraction of the original droplets surface charge. The process of solvent evaporation, droplet contraction and Coulombic explosions is repeated until the molecular adducts are released from the final droplet. If a positive voltage is applied to the capillary, then the droplets will carry positive charges and finally positive ions are formed, such as [M+H]+ and [M+Na]+ adducts. In the negative-ion mode, the base peak is typically the [M-H]- ion. ESI is the softest ionization technique and is well suited for biomolecules, organometallic compounds, non-covalent complexes, gas phase reactivity studies, etc. Advantages of ESI - Soft ionization process so intact molecular ions are observed - ESI allows production of multiply charged ions. This results in the ability of analyzing very high molecular weight species using the most available mass analyzers (e.g. quadrupoles). - ESI is an atmospheric pressure process. This makes it easy to use and easy to interface with HPLC and CE separation techniques. 2 Atmospheric Pressure Chemical Ionization Method APCI sources are very similar in appearance to their ESI counterparts, but the principle is different. Unlike ESI, no voltage is applied to the capillary, but there is a heater for the evaporation of both analytes and mobile phase solvents. After nebulization and solvent evaporation in the heated chamber at about 300 - 500°C, the corona discharge created by the high voltage (ca. 3-4 kV) applied to a needle ionizes first the mobile phase components since they are present at much higher concentration than analytes. The formation of reactant gases is analogous to CI, but occurs at atmospheric pressure. Documentation Finnigan® Then, the ionized mobile phase species react with the analyte by ion-molecule reactions to generate analyte ions. As with ESI and other soft ionization techniques, mostly even-electron molecular adducts are formed, such as [M+H] +, alkali metal adducts [M+Na]+ and [M+K]+ or [M+NH4]+ in case of the positive ion mode and mobile phase containing ammonium additives/buffers. In negative ion mode, [M-H]- is the predominate adduct ion. The relative abundance of fragment ions in the full scan mass spectra is typically negligible, though somewhat higher in comparison to ESI mass spectra. APCI is suitable for compounds ranging from very low to medium polarities with MWs up to 1000 - 2000 Da, which excludes biopolymers (e.g. peptides and proteins), organometallics, and other labile compounds. 3 Ion primary formation N2 + eIon secondary formation N2+. + H2O H2O + H2O+. Proton transfer M + H3O+ N2+. + 2eN2 + H2O+. H3O+ + HO. [M+H]+ + H2O 4