Lab_2 pH Lab-student
advertisement

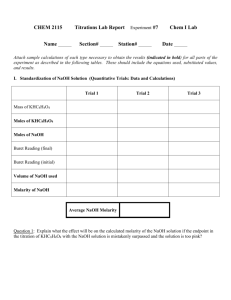
pH Lab MATERIALS 1 micro centrifuge tube rack 10 micro centrifuge tubes 250 mL HCI, 0.1 M 175 mL NaOH, 0.1 M 3 plastic scoopulas 10 dropper pipets Ground antacid samples of Rolaids, Tums, and Alka-Mints Universal indicator Phenolphthalein Electronic Balance with a readability of 0.01 grams pH meter BACKGROUND What is the purpose of an antacid? As their name suggests, antacids work to alleviate the discomfort caused by too much acid working in the stomach and not enough protein for the acid to work on. In the absence of protein (from food), stomach acid will digest stomach tissues, which are composed of protein. Over a prolonged period, this digestive action in the absence of food protein results in a peptic ulcer. A good antacid will maintain the functional pH of the stomach environment, while reducing excess stomach acid. This is achieved as excess stomach acid combines with a base, the active ingredient in antacids, producing a neutralization reaction. An acid is a chemical substance that increases the concentration of hydrogen ion (H+) when dissolved in water. For example, when HCI dissolves in water the following reaction occurs: HCI(aq) H+(aq) + CI-(aq) A base is a chemical substance that increases the concentration of hydroxide ion (OH-) when dissolved in water. For example, when NaOH dissolves in water the following reaction occurs: NaOH(aq)Na+(aq) + OH-(aq) Solutions with a pH less than 7 are acidic. Solutions with a pH greater than 7 are alkaline or basic. The addition of an acid to a basic solution to produce a pH of 7 is called neutralization. Neutralization results in the production of a salt and water, as represented by the following chemical equation: HCI(aq) Acid + + NaOH(aq) Base NaCI(aq) Salt + + H2O(I) Water Pepsin is the primary digestive enzyme found in the stomach, the site of the initiation of protein digestion. Pepsin is an activated form of pepsinogen, a secretion of the chief cells located in the recesses of gastric glands. These glands are widely distributed throughout the walls of the stomach. Inactive pepsinogen is converted to its active form, pepsin, by the presence of hydrochloric acid (HCI), secreted by the parietal cells (also located in the depths of gastric glands). The secretions of these specialized cells, in addition to the slightly alkaline secretions of the mucous cells located in the neck and at the opening of gastric glands, combine to produce what is known as gastric juice, with a pH of approximately 2.0. Heartburn is a burning sensation experienced when stomach contents which have come in contact with gastric juice enter the lower esophagus due to incomplete closure of the lower esophageal sphincter. Acidic gastric juice subsequently causes irritation of the tissues of the esophagus. Continued tissue irritation may eventually lead to an ulcer in this region. Antacids are composed of a variety of bases (brand dependent) designed to neutralize the hydrochloric acid present in gastric juice and thus lessen the burning sensation and discomfort associated with heartburn. A homeostatic system, a feature of most biologic systems, must maintain a specific pH, usually of very limited range, if these systems are to continue operating. Proteins and enzymes (catalytic proteins) operate within specific pH ranges. Outside of these ranges catalytic proteins cease to operate or may even degrade. The optimum pH range for pepsin activity is from 1.5-2.0 depending on the substrate. Therefore, a good antacid will neutralize excess stomach acid while at the same time maintaining a functional environment for digestion. The purpose of the following investigation is to test the neutralizing capabilities of three samples of different antacid brands. LAB PROCEDURE PART I: 1. Label a clean micro centrifuge tube “A”. Carefully pipet 1.0 mL of 0.1 M HCI into this tube. Next, use a clean pipet and add exactly one drop of universal indicator to tube “A”. After closing the lid on the tube, gently agitate it. What happens? Does the solution change color? Place the micro centrifuge tube in your rack as shown in Figure 2. 2. Test same HCl solution ( 3-4mL) with your pH probe Record your observations in the Appropriate space in Data Table 1. 3. Arrange micro centrifuge tubes 1-9 as shown in Figure 2. Using a clean pipet, add 1.0 mL of 0.1 HCI to each of the nine tubes. 4. Add exactly one drop of universal indicator to micro centrifuge tubes 1-9, and record the color of the solution in each tube in Data Table 1 in the column labeled “Univ. Ind”. 5. Now test same HCl solutions (3-4mL) label 1-9 with pH probe and record the your data in Data Table 1 pH Color Transition 2…………………………….Red 3………………………..Red-Orange 4…………………………..Orange 5…………………….…Yellow-Orange 6…………………….……..Yellow 7…………………….……...Green 8…………………….…...Blue-Green 9…………………….……Blue-Gray 10…………………….…….Violet 6. Label a clean scoopula “#1”. Use this scoopula and add one level scoopula of antacid #1 (Rolaids) to micro centrifuge tubes 1, 2, and 3. Close the lids tightly. 7. Label a clean scoopula “#2”. Use this scoopula to add one level scoop of antacid #2 (Tums) to micro centrifuge tubes 4, 5, and 6. Close the lids tightly. 8. Label a clean scoopula “#3”. Use this scoopula to add one level scoop of antacid #3 (Alka-Mints) to micro centrifuge tubes 7, 8, and 9. Close the lids tightly. 9. Agitate each of the tubes to dissolve the antacids. 10. Observe the color changes in each tube (1-9). These changes are directly related to the antacid’s ability to neutralize acid. 11. Now repeat steps 6-10 using your pH probe for beakers 1-9 (3-4mL HCl) adding 3-4 level scoopula of the three antacids and record your findings (in the column labeled “pH+ Antacid”) 12. Record the color of the resulting solution in the column labeled “Univ. Ind. + Antacid” and their approximate pH values (based on a comparison to the pH color chart) in Data Table 1. PART II: CONTROL: 1. Use a measuring pipet to deliver 20 mL of 0.1 HCI into a clean 150-mL beaker. 2. Add three drops of phenolphthalein indicator to the acid. 3. Fill a 25- or 50-mL buret with 0.1 M NaOH. Remove any air bubbles from the tip of the buret by allowing a small volume of solution to flow through the stopcock into an empty beaker. 4. Read the volume at the bottom of the meniscus to the nearest 0.01 mL and record it in Data Table 2 as the initial buret reading. 5. Titrate the 0.1 M HCI with the 0.1 M NaOH until a faint pink color persists for more than 30 seconds. The acid solution should be mixed during the titration by carefully swirling the beaker. 6. Read the volume at the bottom of the meniscus to the nearest 0.01 mL and record it in Data Table 2 as the final buret reading. 7. Repeat steps 1-6 for additional trial, as directed by your teacher. ANTACID 1. Weigh out 0.1 grams of antacid powder and place it in a clean, dry 150-mL beaker. Record the exact mass in the appropriate Data Table. 2. Use a measuring pipet to deliver 20 mL of 0.1 M HCI into the 150-mL beaker. 3. Stir the solution with a glass stirring rod, and then rinse any material that sticks to the stirring rod back into the solution with a small amount of distilled water. 4. Add three drops of phenolphthalein indicator to the acid solution. 5. Fill a 25- or 50-mL buret with 0.1 M NaOH. Remove any air bubbles from the tip of the buret by allowing a small volume of solution to flow through the stopcock into an empty beaker. 6. Read the volume at the bottom of the meniscus to the nearest 0.01 mL. Record this volume as the initial buret reading in the appropriate Data Table. 7. Titrate the acid soluation with the 0.1 M NaOH until a faint pink color persists for more than 30 seconds. The acid solution should be mixed during the titration by carefully swirling the beaker. 8. Read the volume at the bottom of the meniscus to the nearest 0.01 mL. Record this volume as the final buret reading in the appropriate Data Table. 9. Repeat steps 1-8 for each additional trial, as directed by your teacher. Data Table 1 Antacid None Rolaids Tums Alka-Mints Tube No. A 1 2 3 4 5 6 7 8 9 Univ. Ind. Univ. Ind. + Antacid pH pH+Antacid ----------------------------- PART II: Data Table 2: Control Test Results Trial # Final buret reading Initial buret reading Volume of NaOH required Ave. Volume of NaOH 1 2 3 Antacid Test Results Data Table 3 Antacid #1 = Tums 1 2 3 Data Table 4 Antacid #2 = Rolaids 1 2 3 Data Table 5 Antacid #3 = Alka-Mints 1 2 3 Trial # Mass of antacid Final buret reading Initial buret reading Volume of NaOH required Ave. Volume of NaOH Trial # Mass of antacid Final buret reading Initial buret reading Volume of NaOH required Ave. Volume of NaOH Trial # Mass of antacid Final buret reading Initial buret reading Volume of NaOH required Ave. Volume of NaOH QUESTIONS 1. Is the component of an antacid which acts to relieve acid indigestion an acid or a base? List the active ingredients of each of the brands of antacids tested. 2. Which chemical compound is found in all three antacid products? 3. Is carbonate acidic or basic? What is its role in the function of an antacid? 4. Based on the data you listed in Data Table 1, which antacid appears to neutralize the most acid? Support your answer. 5. Using data from Data Tables 2 and 3, what can you conclude about Tums’ neutralizing capabilities? 6. Using data from Data Tables 2 and 4, what can you conclude about Rolaids’ neutralizing capabilities? 7. Using data from Data Tables 2 and 5, what can you conclude about the AlkaMints neutralizing capabilities? This project is funded by a grant awarded under the President’s Community Based Job Training Grant as implemented by the U.S. Department of Labor’s Employment and Training Administration (CB-15-162-06-60). NCC is an equal opportunity employer and does not discriminate on the following basis: against any individual in the United States, on the basis of race, color, religion, sex, national origin, age disability, political affiliation or belief; and against any beneficiary of programs financially assisted under Title I of the Workforce Investment Act of 1998 (WIA), on the basis of the beneficiary’s citizenship/status as a lawfully admitted immigrant authorized to work in the United States, or his or her participation in any WIA Title I-financially assisted program or activity. “This workforce solution was funded by a grant awarded under the President’s Community-Based Job Training Grants as implemented by the U.S. Department of Labor’s Employment and Training Administration. The solution was created by the grantee and does not necessarily reflect the official position of the U.S. Department of Labor. The Department of Labor makes no guarantees, warranties, or assurances of any kind, express or implied, with respect to such information, including any information on linked sites and including, but not limited to, accuracy of the information or its completeness, timeliness, usefulness, adequacy, continued availability, or ownership. This solution is copyrighted by the institution that created it. Internal use by an organization and/or personal use by an individual for non-commercial purposes is permissible. All other uses require the prior authorization of the copyright owner.”