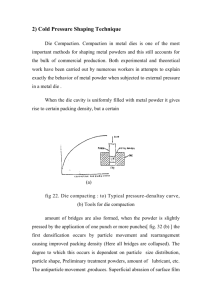
The Compaction of Pharmaceutical Powders
advertisement

http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders The Compaction of Pharmaceutical Powders by Oluwatoyin A. Odeku The compaction properties of pharmaceutical powders are characterised by their compressibility and compactibility. While compressibility is the ability of the powder to deform under pressure, compactibility is the ability to form mechanically strong compacts. Compaction as applicable to a pharmaceutical powder consists of the simultaneous processes of compression and consolidation of a two-phase (particulate solid-gas) system due to an applied force. In the pharmaceutical industry, the effects of such forces are particularly important in the manufacture of tablets and granules, in the filling of hard-shell gelatin capsules and in powder handling in general. The phenomena and mechanisms involved during compaction of pharmaceutical materials have become an important concept in the design and development of solid dosage forms. This is due to the fact that systematic investigations are facilitated by the use of instrumented single-station and multi-station tablet presses, universal testing machines and integrated compaction research systems, also known as compaction simulators. The parameters monitored during compaction vary widely in these studies. Data obtained from the measurements of forces on the punches and the displacement of the upper and lower punches, axial to radial load transmission, die wall friction, ejection forces, temperature changes and other miscellaneous parameters have been used to assess the compaction behavior of a variety of pharmaceutical powders and formulations. More than fifteen different mathematical descriptions of the compaction process have been compiled in the literature and several of these including those of Heckel, Kawakita and Ludde, and Adams have been validated for pharmaceutical systems. Focus is now changing from the mathematical modelling of compressibility to the development and evaluation of a compactibility characteristic where primarily a linear model in the relevant pressure region is investigated. The interesting question whether a correlation between compressibility and compactibility exists is being considered. Introduction Compactibility is the ability of a powder bed to form a mechanically strong tablet; whereas the compressibility is the ability of a powder bed to be compressed and consequently be reduced in volume. The characterisation of powder compression and compaction plays an important role in the manufacturing of tablets and granules, in the filling of hard-shell gelatin capsules and in powder handling in general. Compaction as applicable to a pharmaceutical powder consists of the simultaneous processes of compression and consolidation of a twophase (particulate solid-gas) system due to an applied force (1,2). Compaction of powders is generally used to describe the situation in which these materials are subjected to some level of mechanical force. In the pharmaceutical industry, the effects of such forces are particularly important in the manufacture of tablets. Pharmaceutical powders Powders are subdivided solids which are classified in the British Pharmacopoeia, BP (3) according to the size of their constituent particles which range from less than 1.25 mm to 1.70 mm in diameter. The term ‘powder’ when used to describe a dosage form, however, describes a formulation in which a drug powder has been mixed with other powdered excipients to produce the final product. The function(s) of the added excipients depends upon the intended use of the product (4). Some powders are intended to be used orally e.g. Compound Magnesium trisilicate oral powder BP, and others externally e.g. dusting powder. However, the use of powders as oral dosage forms has been reduced because of problems associated 1 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders with administration of powders which include the undesirability of taking bitter or unpleasant tasting drugs in this manner, the difficulty of protecting from decomposition those powders containing hygroscopic, deliquescent, or aromatic materials, and the time and expense required in the preparation of uniform powders. Hence, the largest use of powder pharmaceutically is in the production of tablets and capsules. Powders intended for compression must possess two essential properties: fluidity and compressibility. Powder fluidity is required so that the material can be transported through the hopper of the tabletting machine and provide adequate filling of the dies to produce tablets of consistent weight and strength. Uneven powder flow can result in excess of entrapped air within powders which in some high speed tabletting conditions may promote capping and lamination. Powder flow can be improved by the incorporation of a glidant such as silicon dioxide, talc, or by making the particles as spherical as possible e. g. by spray-drying. The most popular method of increasing powder flow is by granulation. Icing sugar, for example, has poor flow properties which can be improved by granulation with water (4). Thus, granulation is also the pharmaceutical process that converts a mixture of powders which have poor cohesion, into aggregates capable of compaction. Powder density Bulk density, ρB , is a characteristic of a powder and is given by the mass, M, of a powder occupying a known bulk volume, VB , according to the relationship: ρB = M / VB (1) Bulk density depends on particle shape. As the particle becomes more spherical in shape, bulk density increases (5). The bulk density of a powder bed is always less than the particle or true density (ρT) of its component particles, because the powder contains inter-particle pores or voids. Hence, a powder can possess a single true density but have many bulk densities depending on the way it is packed and the porosity of the powder bed. However, a high bulk density value does not necessarily imply a closed-packed low porosity bed since bulk density is directly proportional to true density. i.e. ρB = ρR . ρT (2) or ρR (3) = ρB / ρT The proportionality constant, ρR , is known as the relative density or packing fraction of the powder bed which is a dimensionless quantity. During compression process, the relative density increases to a maximum of unity when all air spaces have been eliminated. Also: E = 1 - ρR (4) where E is the fractional voidage or porosity of the powder bed which is usually expressed as percentage and termed the powder porosity (6). Compression of powdered solids Compression refers to a reduction in the bulk volume of materials as a result of displacement of the gaseous phase. Stages involved in the bulk reduction of powdered solids are shown in Fig. 1. At the onset of the compression process, when the powder is filled into the die cavity, and prior to the entrance of the upper punch into the die cavity, the only forces that exist between the particles are those that are related to the packing characteristics of the particles, the density of the particles and the total mass of the material that is filled into the die (Fig. 1. I). The packing characteristics of the powder mass will be determined by the packing characteristics of the individual particles (1,2). 2 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders Fig.1: Stages involved in compression (I - III) and decompression. When external mechanical forces are applied to a powder mass, there is usually a reduction in volume due to closer packing of the powder particles, and in most cases, this is the main mechanism of initial volume reduction (Fig. 1. II). However, as the load increases, rearrangement of particles becomes more difficult and further compression leads to some type of particle deformation (Fig. 1. III). If on removal of the load, the deformation is to a large extent reversible, i. e. it behaves like rubber, then the deformation is said to be elastic. All solids undergo elastic deformation when subjected to external forces. With several pharmaceutical materials such as acetylsalicylic acid, elastic deformation becomes the predominant mechanism of compression within the range of maximum force encountered in practice. In other groups of powdered solids, an elastic limit is reached, and loads above this level result in deformation not immediately reversible on the removal of the applied force. Bulk volume reduction in these cases results from plastic deformation and/or viscous flow of particles, which are squeezed into the remaining void spaces, resembling the behaviour of modelling clay. This mechanism predominates in materials in which the shear strength is less than the tensile or breaking strength. Plastic deformation is believed to create the greatest number of clean surfaces. Because plastic deformation is a time dependent process (2,7,8) higher rate of force application should lead to the formation of less new clean surfaces and thus resulting in weaker tablets. Furthermore, since tablet formation is dependent on the 3 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders formation of new clean surfaces, high concentration or over mixing of materials that form weak bonds result in weak tablets. Magnesium stearate for example, forms weak bond and easily wet surfaces. Therefore over mixing of magnesium stearate may lead to weak tablets (2). Conversely, in materials in which the shear strength is greater than the tensile strength, particles may be preferentially fractured, and the smaller fragments then help to fill up the adjacent air spaces. This is most likely to occur with hard, brittle particles and is known as brittle fracture; sucrose behaves in this manner. The ability of a material to deform in a particular manner depends on the lattice structure; in particular whether weakly bonded lattice planes are inherently present. Brittle fracture creates clean surfaces that are brought in intimate contact by applied load. Irrespective of the behaviour of large particles of materials, small particles may deform plastically through a process known as microsquashing, and the proportion of fine powders in a sample may therefore be significant. Asperities that are sheared off larger, highly irregular particles could also behave in this way; hence particle shape is an important factor. Summarily, four stages of events are encountered during compression (1,2): (i) Initial repacking of particles. (ii) Elastic deformation of the particles until the elastic limit (yield point) is reached. (iii) Plastic deformation and/or brittle fracture then predominate until all the voids are virtually eliminated. (iv) Compression of the solid crystal lattice then occurs. Consolidation Consolidation has been described as the increase in the mechanical strength of a material as a result of particle/particle interactions (2). Various mechanisms of powder consolidation are discussed below. When the surfaces of two particles approach each other closely enough (e.g. at a separation of less than 50nm), their free surface energies result in a strong attractive force through a process known as cold welding. The nature of the bonds so formed are similar to those of the molecular structure of the interior of the particle surface (on a molecular scale), but the actual surface area involved may be small. This hypothesis is favoured as a major reason for the increasing mechanical strength of a bed of powder when subjected to rising compressive forces. On the macro scale, most particles encountered in practice have an irregular shape, so that there are many points of contact in a bed of powder. Any applied load to the bed must be transmitted through this particle contacts. However, under appreciable forces, this transmission may result in the generation of considerable frictional heat. If this heat is dissipated, the local rise in temperature could be sufficient to cause melting of the contact area of the particles, which would relieve the stress in that particular region. When the melt solidifies, fusion bonding occurs, which in turn results in an increase in the mechanical strength of the mass. All the deformation effects may be accompanied by the breaking and formation of new bonds between the particles, which give rise to consolidation as new surfaces are pressed together. Another possible mechanism of powder consolidation is asperitic melting of the local surface of powder particles (9,10). During compression, the powder compact typically undergoes a temperature increase usually between 4 and 30 o C, which depends on the friction effects, the specific material characteristics, the lubrication efficiency, the magnitude and rate of 4 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders application of compression forces, and the machine speed (9,11). As the tablet temperature rises, stress relaxation and plasticity increases while elasticity decreases and strong compacts are formed (12). Therefore, compression of material at elevated temperature with increase in ductility should result in stronger tablets (2). Asperitic melting is believed to be important only with relatively low melting point materials for which even very hard asperities are pushed into a more plastic material. The final tablet properties are also affected by the consolidation (i.e. bonding) mechanisms of the powder which is influenced by its chemical nature, the surface area of the contact point, contamination (including film coating, such as magnesium stearate) and interparticulate distance (2). Jones (13) has divided the compression event into a series of time periods, and from this, proposed a number of useful definitions. These are: (i) Consolidation time: time to reach maximum force. (ii) Dwell time: time at maximum force. (iii) Contact time: time for compression and decompression excluding ejection time. (iv) Ejection time: time during which ejection occurs. (v) Residence time: time during which the formed compact is within the die. Fig. 2 is a diagrammatic representation of the lower punch force trace from an eccentric press, and shows Jones' definitions in this context. Dwell time cannot be shown is such a situation, since force reaches a maximum value and then immediately decreases, i.e. a peak is obtained with no plateau. However, in some studies, the maximum force is maintained for prolonged periods and so "dwell time" has a meaning in such circumstances. Furthermore, in rotary presses, a definite though extremely short dwell time is encountered. Fig. 2. Events during the compression process (13). 5 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders Decompression In tabletting, the compression process is followed by a decompression stage, as the applied load is removed. Most compression theories and their related equations describe only the compression stage of the tabletting process, whereas a complete tabletting cycle involves compression, decompression and ejection stages. These theories have in effect proved inadequate to explain some of the compression problems often encountered in routine tablet production. For instance, why some pharmaceutical powders and formulations form crumbly tablets while others form fractured tablets with strong individual pieces; or why minor changes in processing and formulation significantly affect the tabletting characteristics of materials and quality of formed tablets. It is now realized that the decompression stage is as important as (but not independent of) the compression stage in determining whether or not a tablet formulation will form satisfactory tablets. For example, some deformation processes are time-dependent and occur at various rates during the compaction sequence, so that the tablet mass is never in a state of stress/strain equilibrium during the actual tabletting process. This means that the rate at which load is applied and removed may be a critical factor in materials for which dependence on time is significant. More specifically, if a plastically deforming solid is loaded (or unloaded) too rapidly for the process to take place, the solid may exhibit brittle fracture. In view of this, research investigations in recent years have shifted to relating the capping and lamination tendencies of tablet formulations to their plastic and elastic behaviour during the compression/ decompression/ ejection cycle (14-18). The same deformation characteristics that come into play during compression play a role during decompression (2). Decompression leads to a new set of stresses within the tablet as a result of elastic recovery, which is augmented by the forces necessary to eject the tablet from the die. Irrespective of the consolidation mechanism, the tablet must be mechanically strong enough to withstand these new stresses, otherwise structural failure will occur. In particular, the degree and rate of stress relaxation within tablets, immediately after the point of maximum compression have been shown to be characteristic of a particular system (1). This phase of the cycle can provide valuable insight into the reasons behind inferior tablet quality and may suggest a remedy. If the stress relaxation process involves plastic flow, it may continue after all compression force has been removed, and the residual die wall pressure will decay with time. David and Augsburger (19) have been able to interpret plastic flow in terms of viscous and elastic parameters in series. This interpretation leads to the relationship of the form: ln(Ft ) = ln(Fm) - K t (5) where Ft is the force left in the visco-elastic region at time t, Fm is the total magnitude of the force at time t=0 (i.e. when decompression begins) and K is the visco-elastic slope and a measure of the degree of plastic flow. Materials with higher K values undergo more plastic flow and such materials often form strong tablets at relatively low compaction forces. On the other hand, the changing thickness of the tabletting mass due to the compactional force and subsequently due to elastic recovery (ER) during unloading can be used to obtain a measure of plastoelasticity ER/PC; Where PC is the plastic compression of the material under constant load. Force transmission through a powder bed The process of tabletting involves the application of massive compressive forces, which induce considerable deformation in the solid particles (Fig. 3). During normal tablet operations, consolidation is accentuated in those regions adjacent to the die wall, owing to the intense shear to which the material is subjected to, as it is compressed axially and pushed along the wall surface. 6 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders Fig. 3: Diagram of a cross section of a typical single punch and die assembly used for compaction studies. The resistance to differential movement of particles caused by their inherent cohesiveness results in the applied force not being transmitted uniformly throughout the entire mass. In the case of single-station press, the force exerted by the upper punch diminishes exponentially at increasing depths below it. Thus, the relationship between upper punch force, FA , and lower punch force, FL , may be expressed in the form: FL = FA . e - kH/D (6) where k is an experimentally determined material-dependent constant that includes a term for the average die-wall frictional component. H and D are the height and diameter of the tablet respectively. The discrepancy between the two punch forces should be minimized in pharmaceutical tabletting operations, so that there is no significant difference in the amount of compression and consolidation between one region of the tablet and another. The effect of die wall friction can be reduced by having smaller tablet-to-diameter ratios and by adding lubricants (1,20). The distribution of force in an isolated punch and die set is shown in Fig. 4, with force being applied to the top of a cylindrical powder mass. Fig. 4. Force distribution through a powder bed Since there must be an axial (vertical) balance of forces: FA = FL + FD (7) where FA is the force applied to the upper punch, FL is that proportion of it transmitted to the lower punch, and FD is the reaction of the die wall due to the friction at this surface. Because of this inherent difference between the force applied at the upper punch and that affecting material close to the lower punch, a mean compaction force, FM , has been proposed, where: 7 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders FM = (FA + FL ) / 2 (8) FM offers a practical friction-independent measure of compaction load, which is generally more relevant than FA (1). In single-station presses, where the applied force transmission decays exponentially as in equation 6, a more appropriate geometric mean force, FG , might be: FG = ( FA . FL ) 0.5 (9) The use of these parameters is probably more appropriate than the use of F A when determining the relationships between compressional force and such properties as tablet strength. As the compressional force is increased and the repacking of the tabletting mass is completed, the material may be regarded as a single solid body. Then, the compressive force applied in one direction (e.g. vertical) results in a decrease, H, in the height, i.e. a compressive stress. In the case of an unconfined solid body, this would be accompanied by an expansion in the horizontal direction of D. The ratio of these two dimensional changes are known as the Poisson ratio (λ) of the material, defined as: λ = D / H (10) The Poisson ratio is a characteristic constant for each solid material and may influence the tabletting processes. Under the conditions illustrated in Fig 4, the material is not free to expand in the horizontal plane because it is confined in the die. Consequently, a radial diewall force FR develops perpendicularly to the die-wall surface, materials with larger Poisson ratios giving rise to higher values of FR. Classical friction theory can be applied to deduce that the axial frictional force FD is related to FR by the expression: FD = μW . FR (11) where μW is the coefficient of die-wall friction. FR is reduced when materials of small Poisson ratios are used, and in such cases, axial force transmission is optimum. The frictional effects represented by μW arise from the shearing of adhesions that occurs as the particles slide along the die-wall. Hence, its magnitude is related to the shear strength, S, of the particles (or the die-wall-particle adhesions if these are weaker) and the total effective area of contact, Ae, between the two surfaces. Therefore, optimal force transmission is also realized when FD values are reduced to a minimum, which is achieved by ensuring adequate lubrication at the die wall (lower S) and maintaining a minimum tablet height (reducing Ae). A common method of comparing degrees of lubrication has been to measure the applied and transmitted axial forces and determine the ratio FL / FA. This is called the coefficient of lubrication, or R value (1). The ratio approaches unity for perfect lubrication (no wall friction), and in practice, values as high as 0.98 may be realized. Values of R should be considered as relating only to the specific system from which they are obtained, because they are affected by other variables, such as compressional force and tablet H/D (height / diameter) ratio. Compaction data analysis The phenomena and mechanisms involved during compaction of pharmaceutical materials have become an important concept in the design and development of solid dosage forms. This is due to the fact that systematic investigations are facilitated by the use of instrumented single-station and multi-station tablet presses, universal testing machines and integrated compaction research systems, also known as compaction simulators (21-23). The parameters monitored during compaction vary widely in these studies. Data obtained from the measurements of forces on the punches and the displacement of the upper and lower punches, axial to radial load transmission, die wall friction, ejection force, temperature changes and 8 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders other miscellaneous parameters have been used to assess the compaction behavior of a variety of pharmaceutical powders and formulations (24). Many empirical relationships have been proposed to describe the resulting data which may be expressed equivalently in term of stress-strain, pressure-volume or pressure –density since the natural strain, for example, is equal to the natural log of the ratio of the initial bed height or volume to the current height or volume respectively (25). A compaction equation relates some measure of the state of consolidation of a powder, such as porosity, volume (or relative volume), density or void ratio, with a function of the compaction pressure. The initial purpose of fitting experimental data to an equation is usually to linearize the plots so as to make comparisons easier between different sets of data (26). The parameters of the fitting equation can also be used for comparison purposes. A second reason is a practical one of predicting the pressure to obtain a required density. The large range of pressures over which compaction studies can be made, makes it obvious and reasonable to plot pressures logarithmically, so as to spread out the data in a distinguishable form. This was done by Walker (1923) who plotted the relative volume (VR) of the powder compact against the logarithm of the applied axial pressure (Pa) as shown in the following equation (26): VR = a1 – K1 In Pa (12) Later, many other compaction equations have been proposed and today, more than fifteen different mathematical descriptions of the compaction process have been compiled in the literature (24,25) and several of these including those of Heckel (27,28), Kawakita (25) and Adams (29,30) have been validated for pharmaceutical systems. However, it is highly unlikely that a single compaction equation will fit all the compaction mechanisms. In interpreting compaction curves, it is therefore essential to know which mechanisms are operating, or not, over different region of pressure. A good compaction curve should be able to indicate changes in the compression mechanism. 1. Heckel equation Powder packing with increasing compression load is normally attributed to particle rearrangement, elastic and plastic deformation and particle fragmentation as have been previously discussed. The Heckel analysis is a popular method of determining the volume reduction mechanism under the compression force (24, 27, 28) and is based on the assumption that powder compression follows first order kinetics with the interparticulate pores as the reactants and the densification of the powder as the product. According to the analysis, the degree of compact densification with increasing compression pressure is directly proportional to the porosity as follows: dρR / dP = kE (13) where ρR is the relative density at pressure, P, and E is the porosity. The relative density is defined as the ratio of the density of the compact at pressure, P, to the density of the compact at zero void or true density of the material (see equation 3). The porosity (see equation 4) can also be defined as: E = (Vp - V) / Vp = 1 - ρR (14) where Vp and V are the volume at any applied load and the volume at theoretical zero porosity, respectively. Thus, equation 14 can be expressed as: dρR / dP = k ( 1 - ρR ) (15) and then transformed to: 9 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders ln[1 / (1 - ρR )] = k P + A (16) Plotting the value of ln [1 / (1 - ρR )] against applied pressure, P, yields a linear graph having slope, k and intercept, A. The reciprocal of k yields a material-dependent constant known as yield pressure, Py which is inversely related to the ability of the material to deform plastically under pressure. Low values of Py indicate a faster onset of plastic deformation(31). This analysis has been extensively applied to pharmaceutical powders for both single (32,33) and multi-component systems (31,34-38). The intercept of the extrapolated linear region, A, is a function of the original compact volume. It represents two stages of densification - one due to the initial relative density of the powder and the other due to densification by particle rearrangement. From the value of A, the relative density, DA, which represents the total degree of densification at zero and low pressures (31,33,39,40), can be calculated using the equation (41,42): A = ln[1 / (1 - DA )] (17) Thus, DA = 1 - e - A (18) The relative density of the powder bed at the point when the applied pressure equals zero, D0 , is used to describe the initial rearrangement phase of densification as a result of die filling. D0 is determined experimentally and is equal to the ratio of bulk density at zero pressure to the true density of the powder (43). The loose packing of granules at zero pressure tends to yield low D0 values (18, 31). The relative density, DB , describes the phase of rearrangement of particles in the early stages of compression and tends to indicate the extent of particle or granule fragmentation, although fragmentation can occur concurrently with plastic and elastic deformation of the constituent particles (44). The extent of the rearrangement phase depends on the theoretical point of densification at which deformation of particles begins. DB can be obtained from the equation: DB = DA - D0 (19) The particular value of Heckel plots arises from their ability to identify the predominant form of deformation in a material. They have been used: (i) to distinguish between substances that consolidate by fragmentation and those that consolidate by plastic deformation. (ii) as a means of assessing plasticity. Materials that are comparatively soft readily undergo plastic deformation. Conversely, materials with higher mean yield pressure values usually undergo compression by fragmentation first, to provide a denser packing. Hard, brittle materials are generally more difficult to compress than soft ones. Hersey & Rees (45) and York & Pilpel (46) classified powders into three types A, B and C. The classification is based on Heckel plots and the compaction behavior of the material. With type A materials, a linear relationship is observed, with the plots remaining parallel as the applied pressure is increased indicating deformation apparently only by plastic deformation (Fig. 5A). An example of materials that exhibit type A behavior is sodium chloride. Type A materials are usually comparatively soft and readily undergo plastic deformation retaining different degrees of porosity depending on the initial packing of the powder in the die. This is in turn influenced by the size distribution, shape, e. t. c., of the original particles. For type B materials, there is an initial curved region followed by a straight line (Fig. 5B). This indicates that the particles are fragmenting at the early stages of the compression process i. e. brittle fracture preceds plastic flow. Type B Heckel plots usually occur with harder 10 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders materials with higher yield pressures which usually undergo compression by fragmentation first, to provide a denser packing. Lactose is a typical example of such materials. For type C materials, there is an initial steep linear region which become superimposed and flatten out as the applied pressure is increased (Fig. 5C). York and Pilpel (43) ascribed this behavior to the absence of a rearrangement stage and densification is due to plastic deformation and asperity melting. Fig. 5:The 3 different types of Heckel plots. Type A Heckel plots usually exhibit a higher final slope than type B which implies that the former materials have a lower yield pressure. This is so because fragmentation with subsequent percolation of fragments is less efficient than void filling by plastic deformation. 11 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders In fact, as the porosity approaches zero, plastic deformation may be the predominant mechanism for all materials. The two regions of Heckel plots in type B are thought to represent the initial repacking stage and subsequent deformation process, the point of intersection corresponding to the lowest force at which a coherent tablet is formed. In addition, the crushing strength of tablets can be correlated with the values of k of the Heckel plot; larger k values usually indicate harder tablets. Such information can be used as a means of binder selection when designing tablet formulations. Heckel plots can be influenced by the overall time of compression, the degree of lubrication and even the size of the die, so that the effects of these variables are also important and should be taken into consideration. Roberts and Rowe (47) carried out some work involving the application of the Heckel relationship to a wide range of powders. They observed that there was a reasonable agreement between the yield stresses obtained with the Heckel relationship and values measured independently using indentation hardness. The Heckel equations have also proved useful in characterizing the compressional properties of some pharmaceutical excipients developed locally in Nigeria. Itiola (33) showed that three different local starches, namely cassava (from Manihot utilissima ), potato (from Ipomea batatas ) and yam (from Discorea rotundata ) starches deform mainly by plastic flow as has been observed for many official and proprietary starches. This equation has also been used to evaluate the compressional characteristics of tablet formulations containing local gums (31,36,37,48,49) and starches (44) as binding agents. It was believed that Heckel plots generally exhibit linearity for materials at high pressures (24). However, Odeku and Itiola (31) while working on the compressional properties of paracetamol tablet formulations found that the Heckel plots exhibited some degree of linearity at both low and high pressures. Normally, it would be expected that the formulations would consolidate by fragmentation first at low pressures as normally evidenced by an initial curvilinear portion, but the apparent linearity at low pressures suggests that some degree of plastic deformation was also taking place. This is probably due to the fact that the system would start deforming plastically from the moment the yield value for one particle is exceeded during compression. Thus, it should be expected that the process of fragmentation of the granules would occur, to some extent, simultaneously with plastic and elastic deformation of the constituent particles. However, the fact that plasticity is measurable more accurately at higher pressures was evidenced by the higher values of correlation coefficient of the linear second region of the Heckel plots for the formulations. Furthermore, binding agents were shown to facilitate the plastic deformation of pharmaceutical materials with the degree of plasticity increasing with increase in the concentration of the binding agent. With the phenomenon of plastic deformation being timedependent (50,51), the rate of plastic flow may be more important than the total plastic flow for the production of tablets without lamination and capping problems. The rate of deformation could be very important for tabletting considerations considering the fact that most tabletting machines have short dwell or compression time. 2. Kawakita equation The Kawakita equation was developed to study powder compression (25) using the degree of volume reduction, C, a parameter equivalent to the engineering strain of the particle bed and is expressed as: C = (V0 - Vp ) / V0 = a b P / (1+ bP) (20) In practice, the Kawakita equation can be rearranged to give: P / C = P / a + 1 / ab (21) where C is the degree of volume reduction, V0 is the initial volume of the powder bed and V p is the powder volume after compression; a and b are constants which are obtained from the 12 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders slope and intercept of the P/C versus P plots respectively. The constant a is equal to the minimum porosity of the bed prior to compression while b, which is termed the coefficient of compression, is related to the plasticity of the material. Values of 1 – a yield the initial relative density of the material, D I which have been shown to provide a measure of the packed initial relative density of tablets with the application of small pressure or what may be referred to as tapping (31,44,52). The reciprocal of b yields a pressure term, P k , which is the pressure, required to reduce the powder bed by 50% (53,54). The value of P k provides an inverse measurement of plastic deformation during the compression process. The lower the value of P k , the higher the degree of plastic deformation occurring during compression (30,44). Celik (24) reported that the Kawakita equation appeared to be applicable to materials in powder form only. The equation can be adapted by substituting the initial compaction volume with an initial bulk volume in order to have a better fit to the Kawakita equation for granulated materials. Another limitation of the Kawakita equation as reported by Celik (24) is that using this method, the compaction process can be described up to a certain pressure above which the equation is no longer linear. It is now generally accepted that the Kawakita equation is best used for low pressures and high porosities (26). The equation is applied frequently with some success to tapping and vibrational densification (i.e. the higher porosities). In these cases, the pressure term is replaced by the number of taps or time in the case of machine vibration (25). It has been shown that the Kawakita relationship may be employed to determine the tensile strength of agglomerates provided that the influence of the friction at the die wall of the compaction cell has been taken into account since it is well established that wall friction significantly increase resistance to deformation (25,30). Kawakita and Ludde stated that the equation holds best for soft fluffy pharmaceutical powders and stated that particular attention must be paid to the measurement of the initial volume, Vo, and that deviation from this equation is sometimes due to the measured value of Vo. The Kawakita equation however, has also been applied to granules which cannot be described as light and fluffy (31,44,48). Odeku and Itiola (31) have shown that the pressure term, P k provides a measure of the total amount of plastic deformation occurring during compression. Recent studies carried out in our laboratory have shown that the tensile strength of paracetamol tablets containing pigeon pea, plantain and corn starches as binding agent, was inversely related to the values of P k values of the various formulations (55). This supports the assertion that as has been previously established that higher total plastic deformation would lead to more contact points for interparticulate bonding to produce stronger tablets (30,44,56). The Heckel and Kawakita plots have been employed to evaluate and describe the compressional characteristics of paracetamol formulations (31). Both plots have their limitations and are believed to generally exhibit linearity for materials at high and low pressures, respectively (24). Thus both plots have been used with the hope of obtaining more accurate information on the compressional characteristics of the paracetamol tablet formulations. Research has shown that the Heckel and Kawakita plots gave largely different indications for the plasticity of the formulations (31). The observed differences between P y and P k for the different binders are probably due to the fact that the P y values relate essentially to the onset of plastic deformation during compression while the P k values appear to relate to the amount of plastic deformation occurring during the compression process, especially with plastic deformation being a time-dependent phenomenon (50,51). Thus, the more the time allowed for plastic deformation to occur (i. e. increase in dwell time), the more the difference between the values of P y and P k would be, although it should be remembered that for every material, there would be a limiting dwell time after which no more plastic deformation would occur. Furthermore, it should be noted that differences between P k and P 13 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders would be observable more for materials that deform mainly by plastic flow than for those that deform mainly by fragmentation (47). y It should be possible, therefore, to obtain more information on the deformation profile of a material from the combined use of P k and P y . For example, to obtain optimum plasticity, there may not be much point in using a long dwell time for a material with a low P y but a high P k . On the other hand, it should be of significant benefit to use a long dwell time for a material with a high P y but low P k values. However, material with low P y and P k values should not give any appreciable problems on any type of tabletting machine, while materials with a combination of high P k and P y values would give compressional problems on virtually any type of tabletting machine, and reformulation or the addition of plastic materials may be necessary in such cases. 3. Adams Equation The Adams equation (29,30) was derived in order to estimate the fracture strength of single granules from in-die compression data. It models the bed of granules in the die as a series of parallel load-bearing columns. The following equation was derived: In P = In (тo’ / α’ ) +α’ ε + In (1 – e (-α’ε) ) (22) where P is the applied pressure and ε is the natural strain which is given by: ε = In (Ho / Hp ) (23) where Ho and Hp are the initial and current height of the bed respectively. The quantity тo’ is the apparent single agglomerate strength which is related to the actual strength, тo , as follows: тo’ = k1 тo (24) where k1 is a constant. The quantity α ’ is related to the pressure coefficient, α of the agglomerate strength by the following expression: α ’ = k2 α (25) where k2 is a constant. At higher values of natural strain, the last term of the Adams equation becomes negligible and can be omitted, leaving a linear function. The intercept and slope of this linear part of the profile were used to calculate the compression parameter тo’ . Nicklasson & Alderborn (56) have used the Adams and Kawakita equations to analyse the compression mechanics of pharmaceutical agglomerates. They found that both 1/ b (Pk ) from Kawakita and т o ’ from Adams were related with agglomerates porosity and composition. The two parameters were related to the intergranular pore structures and tensile strength of the tablets formed from the agglomerates. They concluded that 1/ b and тo’ may be interpreted as measure of agglomerate shear strength during uniaxial confined compression and as such may be used as indications of tabletting performance of the agglomerates. Conclusion The understanding of the principles involved in compressibility and compactibility are required to characterise the compaction profiles of pharmaceutical materials. Both phenomena are important in the tabletting of the materials. The importance of each will depend largely on the type of compact required i.e. whether soft or hard and on the brittle properties of the materials. Various mathematical equations have been used to describe the compaction process. The use of one single equation is unlikely to be adequate since different materials consolidate by different mechanism depending on their properties. Some progress has been made in this direction with prominence of some relevant parameters obtained from the more useful compression equations. The rate and extent of plastic deformation are now 14 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders more completely characterized by the combined use of P y and P k from the Heckel and Kawakita equations respectively. There is also promise of additional indices from newer approaches to the study of the compression process. More work is however required. References 1. Marshall, K., Compression and consolidation of powdered solids. In : The Theory and Practice of Industrial Pharmacy. Lachman, L., Lieberman, H. A. and Kanig, J. L. (Eds.) 3 rd Edition (1986) Lea & Febiger, Philadelphia , pp. 66-99. 2. Bodga, M. J. (2002) Tablet compression: Machine theory, design and process troubleshooting. In: Encyclopedia of Pharmaceutical Technology. Swarbrick J and Boylan J (Eds). Marcel and Dekker Inc., USA . Vol. 3: 2669 – 2688. 3. British Pharmacopoeia (1998) HMSO, London . 4. Summers, M. P. Granulation. In : Pharmaceutics: The science of dosage form design. Aulton, M. E. (Ed.) ELBS. 1 st Edition (1988), Longman grp. Ltd., U. K. pp. 616-628. 5. Banker, G. S. and Anderson, N. R., Tablets. In : The Theory and Practice of Industrial Pharmacy. Lachman, L., Lieberman, H. A. and Kanig, J. L. (Eds.) 3 rd Edition. (1986) Lea & Febiger, Philadelphia , pp. 301-303. 6. Staniforth, J. N. Powder flow. In : Pharmaceutics: The science of dosage form design. Aulton, M. E. (Ed.) ELBS. 1 st Edition (1988), Longman grp. Ltd., U. K. pp. 600-615. 7. Armstrong N. A. (1989) Time dependent factors involved in powder compression and tablet manufacture. Int. J. Pharm. 49: 1-13. 8. Ayorinde, J. O. Odeku, O. A. and Itiola, O. A. (2005) The survival of B. Subtilis spores in dicalium phosphate, lactose and corn starch and their binary mixtures during tableting. Pharmaceutical Technology 29 (12):56-67. 9. Rankell, A. S. and Higuchi, T. (1968) Physics of tablet compression: XV. J. Pharm. Sci., 57: 574577. 10. York, P. and Pilpel, N. (1973) The tensile strength and compression behaviour of lactose, four fatty acids and their mixtures in relation to tabletting. J. Pharm. Pharmacol., 25: 1P-11P. 11. Hanus, E. J. and King, L. D. (1968) Thermodynamic effects on the compression of solids. J. Pharm. Sci., 57: 677-684. 12. Esezobo, S. and Pilpel, N. (1986) The effect of temperature on the plasto-elasticity of some pharmaceutical powders and on the tensile strength of their tablets. J. Pharm. Pharmacol., 38: 409-413. 13. Jones, T. M. (1981) The physicotechnical properties of starting materials used in tablet formulation. Int. J. Pharm. Prod. Manuf., 2: 17-24. 14. Carless, J. E. and Leigh, S. (1974) Compression characteristics of powders: Radial die wall pressure transmission and density changes. J. Pharm. Pharmacol., 26: 189-297. 15. Hiestand, E. N., Wells, J. E., Poet, C. B. and Ochs, J. F. (1977) Physical processes of tabletting. J. Pharm. Sci. 66 : 510 - 519. 16. Rees, J. E. and Rue, P. J. (1978) Time-dependent deformation of some direct compression excipients. J. Pharm. Pharmacol. , 30: 601-607. 17. Krycer, I. , Pope, D. G. and Hersey, J. A. (1982) The role of intragranular porosity in powder compaction. Powder Technol., 33: 101-111. 18. Itiola, O. A. and Pilpel, N. (1986) Tabletting characteristics of metronidazole formulations. Int. J. Pharm. 31: 99-105. 19. David, S. T. and Augsburger, L. L. (1977) Plastic flow during compression of directly compressible fillers and its effect on tablet strength. J. Pharm. Sci., 66: 155-159. 15 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders 20. Briscoe B. J. and rough S.L. (1998) The effects of wall friction on the ejection of pressed ceramic parts. Powder Technol. 99: 228- 233. 21. Higuchi, T., Rao, A. N., Busse, L. W. and Swintosky, J. V. (1953) The Physics of tablet compression, 2: the influence of degree of compression on properties of tablets. J. Am. Pharm. Assoc. Sci. Ed. 42: 194-200. 22. Higuchi, T., Nelson, E. and Busse, L. W. (1954) The physics of tablet compression, 3: design and construction of an instrumented tabletting press. J. Am. Pharm. Assoc. Sci. Ed. 43: 344-348. 23. Train, D. (1954) An investigation into compaction of powders. J. Pharm. Pharmacol. 8: 745-761. 24. Celik, M. (1992) Overview of compaction data analysis techniques. Drug Dev. Ind. Pharm., 18: 767-810. 25. Kawakita, K. and Ludde, K. H. (1970/71) Some considerations on powder compression equations. Powder Technol., 4: 61-68. 26. Denney P.J. (2002) Compaction equations: a comparism of the Heckel and Kawakita equations Powder Technol. 127: 162 – 172. 27. Heckel, R. W. (1961) Density-pressure relationships in powder compaction. Trans. Metall. Soc. AIME., 221: 671-675. 28. Heckel, R. W. (1961) An analysis of powder compaction phenomena. Trans. Metall. Soc. AIME., 221: 1001-1008. 29. Adams , M. J. Mullier M. A. and Bircall, J. D. (1994) Agglomerates strength measurements using a uniaxial confined compression test. Powder Technol . 78: 5-13. 30. Adams, M. J. and McKeown, R. (1996) Micromechanical analysis of the pressure-volume relationships for powders under confined uniaxial compression. Powder Technol . 88: 155-163. 31. Odeku, O. A and Itiola O. A. (1998) Evaluation of khaya gum as a binder in a paracetamol tablet formulation. Pharm Pharmacol Commun ., 4: 183-188. 32. Duberg, M. and Nystrom, C. (1986) Studies on direct compression of tablets. XVII. Porositypressure curves for the characterization of volume reduction mechanisms in powder compression. Powder Technol. , 46: 67-75. 33. Itiola, O. A. (1991) Compressional characteristics of three starches and the mechanical properties of their tablets. Pharm. World J., 8(3) : 91-94. 34. Kurup, T. R. R. and Pilpel, N. (1978) Compression characteristics of pharmaceutical powder mixtures. Powder Technol., 19: 147-155. 35. Garr, J. S. M. and Rubinstein, M. H. (1992) Consolidation and compaction characteristics of three-component particulate system. Int. J. Pharm., 82: 71-77. 36. Adeyemo, O. A. and Itiola, O. A. (1993) Effects of khaya gum and gelatin on the compressional characteristics of a griseofulvin tablet formulation. J. West Afr. Pharm . 7: 27-29s 37. Odeku, O. A. and Itiola, O. A. (2002) Characterisation of khaya gum as a binder in a paracetamol tablet formulation. Drug Dev. Ind. Pharm. 28 (3); 329-337 38. Odeku, O. A., Itiola O. A. and Adeniran, A. A. (1998) Effects of Yam and Corn Starches on the Mechanical and Disintegration of Paracetamol Tablets. Proceedings of the 1 st International Workshop on Herbal Medicinal Products , pp. 193-200 39. Paronen, P. and Juslin, M. (1983) Compressional characteristics of four starches. J. Pharm. Pharmacol., 35: 627-635. 40. Mitrevej, A., Faroongsarng, D. and Sinchaipanid, N. (1996) Compression behaviour of spray dried rice starch. Int. J. Pharm., 140: 61-68. 41. Humbert-Droz, P., Gurny, R., Mordier, D. and Doelker, E. (1983) Densification behaviour of drugs presenting availabilty problems. Int. J. Pharm. Tech, Prod. Mfr., 4: 29-35. 16 http://www.pharmainfo.net/reviews/compaction-pharmaceutical-powders 42. Roberts, R. J. and Rowe R.C (1985) The effect of punch velocity on the compaction of a variety of materials. J. Pharm. Pharmacol., 37: 377 - 384. 43. Chowhan, C. T. and Chow, Y. P. (1981) Compression behaviour of granulations made with different binders. Int. J. Pharm. Tech. Prod. Mfr. 2(1) : 29-34. 44. Odeku, O. A., Awe, O. O., Popoola, B. Odeniyi, M. A. and Itiola, O. A. (2005) Compression and mechanical properties of tablet formulations containing corn, sweet potato, and cocoyam starches as binders. Pharm. Tech. 29 (4): 82-90. 45. Hersey, J. A. and Rees, J. E. (1971) Deformation of particles during briquetting. Nature, 230: 96. 46. York, P. and Pilpel, N. (1972) The effect of temperature on the mechanical properties of some pharmaceutical powders in relation to tabletting. . J. Pharm. Pharmacol., 24: 47P-56P. 47. Roberts, R. J. and Rowe, R. C. (1986) The effect of the relationship between punch velocity and particle size on the compaction behaviour of materials with varying deformation mechanisms. J. Pharm. Pharmacol. , 38: 566-571. 48. Odeku, O. A. (2005) Assessment of Albizia zygia gum as binding agent in tablet formulations. Acta Pharm. 55 (3):263-276. 49. Odeku, O. A. and Patani, B. O. (2005) Evaluation of dika nut mucilage (Irvingia gabonensis) as a binder in metronidazole tablet formulations. Pharm. Dev. Tech. 10: 439-446. 50. Rees, J. E. and Rue, P. J. (1978) Time-dependent deformation of some direct compression excipients. J. Pharm. Pharmacol. , 30: 601-607. 51. Akande, O. F., Ford, J. L. Rowe, P. H. and Rubinstein, M. H. (1998) The effects of lag-time and dwell-time on the compaction properties of 1:1 paracetamol/microcrystalline cellulose tablets prepared by pre-compression and main compression. J. Pharm. Pharmacol. , 50: 19-28. 52. Podczeck, F. and Sharma, M. (1996) The influence of particle size and shape of components of binary powder mixtures on the maximum volume reduction due to packing. Int. J. Pharm., 137: 41-47. 53. Shivanand, P. and Sprockel, O. L. (1992) Compaction behaviour of cellulose polymers. Powder Technol., 69: 177-184. 54. Lin, C. and Cham, T. (1995) Compression behaviour and tensile strength of heat-treated polyethylene glycols. Int. J. Pharm., 118: 169-179. 55. Dare, K., Akin-Ajani, D. O., Odeku, O. A., Odusote O. M. and Itiola, O. A. (2006) Effects of pigeon pea and plantain starches on the compressional, mechanical and disintegration properties of paracetamol tablets. Drug Dev. Ind. Pharm . 32: (In Press) 56. Nicklasson, F and Alderborn, G. (2000) Analysis of the compression mechanics of pharmaceutical agglomerates of different porosity and composition using the Adams and Kawakita equation. Pharm. Res. 17 (8): 949-954. 17