Periodic Trends in Reactivity Lab
advertisement
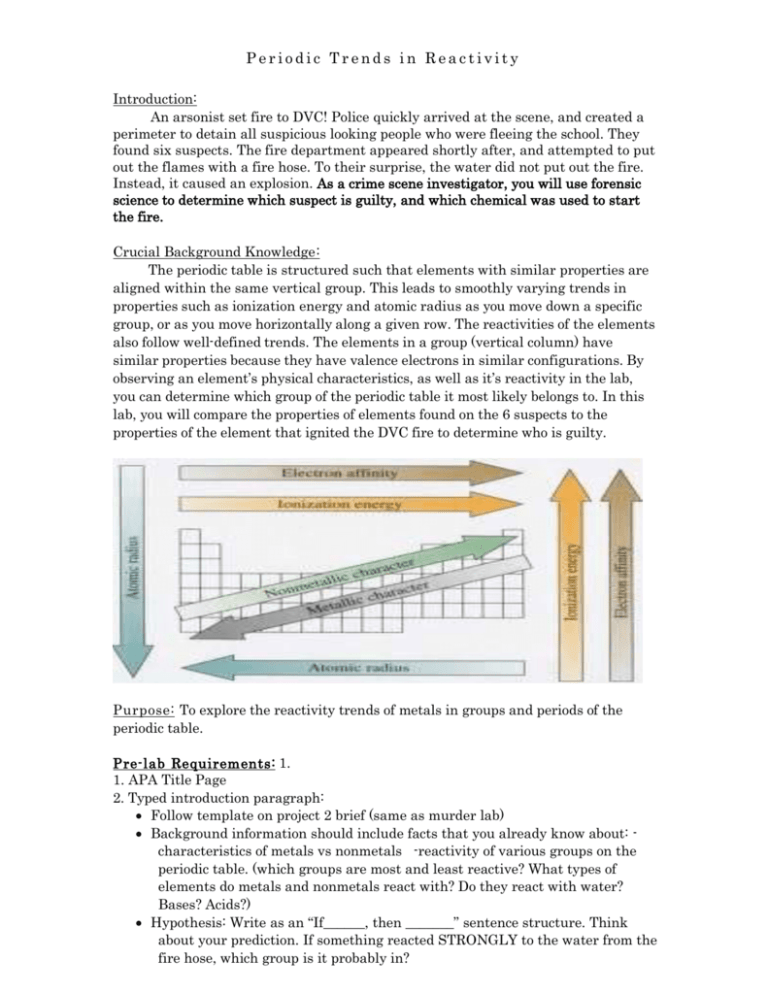
Periodic Trends in Reactivity Introduction: An arsonist set fire to DVC! Police quickly arrived at the scene, and created a perimeter to detain all suspicious looking people who were fleeing the school. They found six suspects. The fire department appeared shortly after, and attempted to put out the flames with a fire hose. To their surprise, the water did not put out the fire. Instead, it caused an explosion. As a crime scene investigator, you will use forensic science to determine which suspect is guilty, and which chemical was used to start the fire. Crucial Background Knowledge : The periodic table is structured such that elements with similar properties are aligned within the same vertical group. This leads to smoothly varying trends in properties such as ionization energy and atomic radius as you move down a specific group, or as you move horizontally along a given row. The reactivities of the elements also follow well-defined trends. The elements in a group (vertical column) have similar properties because they have valence electrons in similar configurations. By observing an element’s physical characteristics, as well as it’s reactivity in the lab, you can determine which group of the periodic table it most likely belongs to. In this lab, you will compare the properties of elements found on the 6 suspects to the properties of the element that ignited the DVC fire to determine who is guilty. P u r p o s e : To explore the reactivity trends of metals in groups and periods of the periodic table. P r e - l a b R e q u i r e m e n t s : 1. 1. APA Title Page 2. Typed introduction paragraph: Follow template on project 2 brief (same as murder lab) Background information should include facts that you already know about: characteristics of metals vs nonmetals -reactivity of various groups on the periodic table. (which groups are most and least reactive? What types of elements do metals and nonmetals react with? Do they react with water? Bases? Acids?) Hypothesis: Write as an “If______, then _______” sentence structure. Think about your prediction. If something reacted STRONGLY to the water from the fire hose, which group is it probably in? Periodic Trends in Reactivity Sample hypothesis sentence frames: “If the six elements in question are reacted with water, then the element in group ________ will react most vigorously.” “If the six elements in question are reacted with water, then the (metals/nonmetals/metalloids) will have the weakest reaction.” This means that they could not have exploded upon contact with water from the fire hose, and that their owners are innocent. “If the six elements are reacted with water, ____________ will have the strongest reaction, but ______________ will not react at all. 3. Methods paragraph (typed) Do your best! This is a rough draft that you will be able to edit later. It is useful to write it ahead of time to get a jump start on the write up, and to familiarize yourself with the procedure you will be performing. Write it by reading the procedures (for all 3 parts) in this lab and summarizing the key points. When you edit later, be sure to include how many g of each metal you tested, what glassware/equipment you used (with size in mL), and how many trials you performed Periodic Trends in Reactivity Procedures: Part 1: Trends in Physical Characteristics 1. Obtain samples of the 6 elements that were confiscated from the suspects. 2. Observe each element, and record qualitative data below. Describe physical characteristics such as color, texture, shape, and malleability. DO NOT touch any elements with your hands! They could be dangerous! Instead, use forceps or a metal spatula. Data Part 1: Table 1: Physical Characteristics of Suspicious Elements Element UNKNOWN substance #1 Observations: Physical Characteristics Magnesium Calcium UNKNOWN substance #2 UNKNOWN Substance #3 Tin PART 1 ANALYSIS: 1. Look at the periodic table. Are magnesium, calcium, and tin classified as metals, nonmetals, or metalloids? Do the physical characteristics that you just observed match the ones that we learned in class? Explain why, using evidence from table 1 to support your claim. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Periodic Trends in Reactivity 2. Based on your observations of the 3 unknown substances, which ones do you think are metals? Nonmetals? Metalloids? Explain, using evidence from table 1 to support your claim. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Part 2: Reactivity in Water Procedures: 1. Your instructor will do a demonstration of unknown substance 1’s reaction with water. Record observations in the table below. 2. Place a small sample of magnesium in a test tube and cover with water. Record observations. * Make sure you use a new, clean test tube for each chemical to avoid cross contamination and dangerous reactions. 3. Place a small sample of calcium in a test tube with several milliliters of water. Record observations. 4. Place a small sample of unknown substance #2 in a test tube and cover with water. Look closely and record observations. 5. Place a small sample of tin in a test tube and cover with water. Look closely and record observations. 6. Place a small sample of unknown substance #3 in a test tube and cover with water. Look closely and record observations. Periodic Trends in Reactivity Data Part 2 Table 2: Reactivity in Water Metal UNKNOWN substance #1 Obs ervat i on s: Rea ct ivi t y i n wat er Magnesium Calcium UNKNOWN substance #2 Tin UNKNOWN Substance #3 ANALYSIS PART 2: 1. What might be a reason for the difference in the way magnesium and calcium react in water? (Hint: Look at their locations on the periodic table. Lithium, sodium, and potassium are also progressively more reactive). ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ 2. Why do you think that tin was unreactive in water? (hint: think about what group it is in on the periodic table) ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Periodic Trends in Reactivity 3. Explosions are chemical changes, because they produce gas and release heat. Hence, the fiery DVC explosion was caused by an element chemically reacting with water. Based on your observations from Table 2, which elements could have caused the explosion (if a large amount was used)? Which suspects might be guilty? Which suspects are most likely innocent? Explain, using evidence from table 2 to support your claim. ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ ______________________________________________________________________________ Part 3 - Reactivity in HCl Although 4 of the subjects are probably innocent, you decide that it’s a good idea to determine what unknown elements that they are carrying before releasing them from custody. 1. Obtain a small sample of magnesium, unknown substance #2, tin, and unknown substance #3. You may reuse the Mg from Part 1. Carefully dump out the water from the test tube containing the magnesium but keep the magnesium in the test tube. 2. Place each sample in a separate tube. 3. Add a small amount of dilute HCl to each test tube, just enough to cover the sample. Record your observations. Part 2 – Reactivity in HCl Metal Magnesium UNKNOWN substance #2 Tin UNKNOWN Substance #3 O b s e r v a t i o n s : R e a c t i o n i n HCl Periodic Trends in Reactivity ANALYSIS PART 3 1. Put the 4 elements from part 2 in order, from least to greatest reactivity. 2. Using your data from parts 1,2, and 3, try to determine what the 3 unknown elements are. Explain your guesses, with evidence. (example: Unknown substance #1 is probably a metal/nonmetal/metalloid, because it had a shiny, silver appearance (table 1). Furthermore, unknown substance 1 is probably in group ____ on the periodic table, because it reacted very strongly/weakly with water (table 2). Unknown substance 1 could be (element) because it was more reactive than the known alkaline earth metals. (element) shows this relative reactivity because it is located to the left of the alkaline earth metals on the periodic table.) Unknown substance 1: _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Unknown substance 2: _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Unknown substance 3: _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ Periodic Trends in Reactivity 3. Reactivity INCREASES/DECREASES as you move down a group, and INCREASES/ DECREASES as you move across a period. (circle the correct trend). 4. Although unknown substance 1 seems to be the most reactive in water, Calcium reacted strongly as well. If you were able to collect a small amount of metal from the crime scene, what additional tests could you perform to see if it were calcium or unknown substance 1? _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ 5. What are some pros for using reactivity to identify unknown substances? What are some cons? _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ _________________________________________________________________________________ 6. A) Based on your complete lab results, which suspect do you think is guilty? Which element do you think the suspect used to burn down DVC? ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ B) Would you feel comfortable sending a suspect to jail based on your lab results? Why or why not?_____________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ ________________________________________________________________________________ Periodic Trends in Reactivity _______________________________________________________________________________ Periodic Trends in Reactivity






