Modeling Alkanes Lab
advertisement

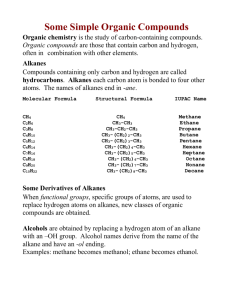
Modeling Alkanes Lab PRELAB Methane (CH4) is the simplest hydrocarbon. It is the first in a series of hydrocarbons known as alkanes. Each carbon atom in an alkane forms single covalent bonds with four other atoms. Alkanes are considered saturated hydrocarbons because each carbon atom is bonded to the maximum number of other atoms (four). Electron-dot (Lewis Dot) structures and structural formulas represent a 2-dimensional view of compounds which only shows what atoms are bonded to what atoms. We will now make models of alkanes to see their 3-dimensional geometry. PROCEDURE 1. Assemble a model of methane. Use yellow balls for hydrogen and black balls for carbon. Draw the LD structure and structural formula for methane. a. How does your model compare with the electron-dot and structural formulas? b. The actual shape of a methane molecule is a tetrahedron with angles of 109.5o instead of 90o as the structural formula shows. Notice that the tetrahedron shape allows the electron pairs to remain as far away from each other as possible. c. Sketch your 3-dimensional model. 2. Assemble models of a two-carbon and a three-carbon alkane molecule. Recall that each carbon atom in an alkane is bonded to four other atoms. Set up the following data table to compare your two models. DATA TABLE 1 carbon model Sketch of model The # of hydrogen Atoms in molecule Electron dot structure Structural formula Molecular formula 2 carbon model 3 carbon model Additional notes Naming Alkanes When naming alkanes, the root is followed by the suffix –ane (designating an alkane). The root refers to the number of carbon atoms in the backbone carbon chain. Example: ethane eth – means 2 carbons - ane means alkane The 2 carbons are connected and hydrogen atoms surround the carbons so that each carbon has 4 bonds. You can use the following formula to predict how many hydrogens are needed with so many carbons: CnH2n + 2 n = the number of carbon atoms A four-carbon alkane: n = 4, so the molecular formula would be C4H2(4) + 2 or C4H10. The long version of the formula is CH3CH2CH2CH3. Make this model. ANALYSIS Complete the following table in your lab book: Alkane Molecular Formulas Name # of carbons short version long version Methane 1 CH4 CH4 Ethane 2 Propane 3 Butane 4 Pentane 5 Hexane 6 Heptane 7 Octane 8 Nonane 9 Decane 10 1. Write the structural formulas for butane and pentane. 2. What do you notice about the prefixes for alkanes with 5 – 10 carbons? 3. Name the alkanes with these formulas: a. CH3 CH2 CH2 CH2 CH2 CH2 CH3 b. CH3 CH2 CH2 CH2 CH2 CH2 CH2 CH2 CH3 c. Write the shorter molecular formulas for the two alkanes described in 2a and 2b. 4. Write the molecular formula for an alkane containing 25 carbon atoms. Did you decide to write a short version or a long version of the formula for this compound? Why?