1.1.1 Guidelines for registration of Herbicides u/s 9(3B)
advertisement

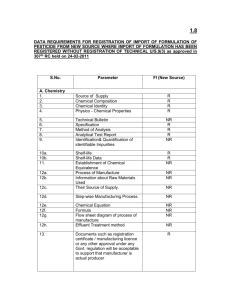
1.1.1 GUIDELINES / DATA REQUIREMENTS FOR REGISTRATION OF HERBICICIDES U/s 9(3) / 9(3B) / 9(4) as on 05-10-2011 Abbreviations : : R : Required NR TIM : Technical Indigenous Manufacture TI : FI : Formulation Import FIM : IM AR : : Indigenous Manufacture Already Registered NF : Not Required Technical Import Formulation Indigenous Manufacture New Formulation Note: (i) For Import of Formulation u/s 9(3): (ia) If the applicant does not seek registration for import of technical together with formulation in such a case the applicant shall be required to submit complete data as per the requirement of technical Import in addition to formulation import. The Deemed Registered Status of “technical” in such cases shall come into force only after expiry of 3 years from the date of registration of its ”formulation” for import and that the norm shall be applicable to all the formulations” which are already registered for import without registering the “technical”; and in cases where registration granted for formulations under Section 9(3B) with commercialization, the period of three years for effecting the deemed registration of “technical” will be computed from the date of issuing registration for formulation under Section 9(3B) with commercialization. The requirements of data on technical w.r.t. shelf-life & informations on packaging, as per decision of 313 th RC held 8th Nov., 2010, are under Appeal U/s 10 of the Insecticides Act, 1968. . (ib) Rationale for importing formulation. (ii) If the applicant does not seek registration for indigenous manufacture of technical together with Formulation, in such a case the applicant shall be required to submit complete data as per the requirement of Technical Indigenous Manufacture in addition to formulation indigenous manufacture subject to fulfillment of condition that the technical and formulation products will be manufactured in the same premises. Deemed registration status of technical shall come into force after expiry of three years from registration of formulation for indigenous manufacture without registration of technical U/s 9(3) and other conditions regarding submission of data on shelf-life & information on packaging etc. shall be same as for the cases for import of formulation without registration of technical (As per decisions of 272nd (17-01-2007) & 314th (24/27-01-2011) RC meetings. (iii) Data requirements of chemical pesticides are also applicable for registration of chemical plant growth regulators (PGR). 1 S. No. 9(3B) Parameter TI 1 2 9(3) TIM FIM TI 3 4 5 TIM FI 6 7 FIM 8 9(4) TI TIM TI Vs Vs (New Source) TIM TI 9 10 11 NF** (IM) TIM * (AR) Vs TI 12 A. Chemistry 1. Source of Supply of Technical R NR R R NR R R R NR R R NR 2. Chemical Composition R R R R R R R R R R R R 3. Chemical Identity of technical R R R R R R R R R R R R 4. Physico - Chemical Properties of adjuvants R R R R R R R R R R R R 5. Technical Bulletin R NR NR R NR R NR R NR R R NR 6. Specification R R R R R R R R R R R R 7. Method of Analysis R R R R R R R R R R R R 8. Analytical Test Report R R R R R R R R R R R R 9. Identification& Quantification of identifiable Impurities NR NR NR R R NR NR R R R NR R 10a. Shelf-life clRm R R R R R R R R R R R R 10b. Shelf-life Data NR NR NR R R R R R R R R NR 11. Establishment of Chemical Equivalence NR NR NR NR NR NR NR R R NR NR R 12a. Process of Manufacture NR R R NR R NR R NR R NR R R 12b. Information about Raw Materials Used NR R R NR R NR R NR R NR R R 12c. Their Source of Supply. NR R R NR R NR R NR R NR R R 12d. Step-wise Manufacturing Process. NR R R NR R NR R NR R NR R R 12e. Chemical Equation NR R NR NR R NR NR NR R NR NR R 12f. Formula NR R NR NR R NR NR NR R NR NR R 12g. Flow sheet diagram of process of manufacture NR R R NR R NR R NR R NR R R 12h. Effluent Treatment method NR R R NR R NR R NR R NR R R 13. Documents such as registration certificate / Certificate of DNA/manufacturing R NR NR R NR R NR R NR R NR NR 2 licence or any other approval under any Govt. regulation will be acceptable to support that manufacturer is actual producer 14. Certificate from R manufacturer that the dealer/ trader is an authorized dealer/ trader of the manufacturer. NR NR R NR R NR R NR NR NR NR 15. A test report about the R quality of the product from a laboratory as per GLP scheme or from a company of ISO-9000. This requirement will be provided along with first consignment. Thereafter, each consignment should have proper analytical test report of the manufacturer. NR NR R NR R NR R NR NR NR NR 16. The applicant should R provide sample along with standards technical sample from the principals/ authorized dealers for chemical verification. In case of technical grade pesticides u/s 9(3), samples of std. impurities are also to be provided for chemical verification. In process sample to be provided in case of indigenous manufacture of technical u/s 9(3) TIM & 9(4) TIM with undertaking R R R R R R R R R R R 17. Methodology for residue R estimation as per BIS format. R R R R R R R R R R NR B. BIOEFFICACY- As approved in 291st RC extended meeting held on 28-07-2008 18a. Bio-effectiveness NR NR R* NR R** R** R** R+ R+ R++ R** NR 18b. Phytotoxicity NR NR R* NR R** R** R** R+ R+ R++ R** NR 19. Translocation in plants R R NR R R NR NR NR NR NR NR NR 3 20. Metabolism in soil R R NR R R NR NR NR NR NR NR NR 21. Metabolism in water R R NR R R NR NR NR NR NR NR NR 22. Metabolism in plant R R NR R R NR NR NR NR NR NR NR 23. Persistence in soil R R R R R R R NR NR NR R NR 24. Persistence in water R R R R R R R NR NR NR R NR 25. Persistence in plant R R R R R R R NR NR NR R NR 26. Compatibility with other NR chemicals NR R# NR NR R# R# NR NR NR R NR 27. Residues in plant NR NR R* NR NR R** R** R^ R^ R++ R**/ NR NR 28. Residues in soil NR NR R* NR NR R** R** NR NR NR NR NR 29. Residue tolerance limits NR fixed by foreign countries NR R NR NR R R NR NR NR R NR 30. Cost benefit ratio NR NR NR NR NR NR NR NR NR NR NR NR 31. Registration status in foreign countries R NR R R NR R R NR NR NR R NR R* : Two seasons/years data generated at minimum two agroclimatic conditions/locations+ R** : Two seasons/years data generated at minimum three agroclimatic conditions/locations+ Locations+ : Locations shall be applicable for the crops for which required agroclimatic conditions are not avRlable. R #: Data on compatibility is required, if the product is proposed to mix with other chemicals. Notes: (i) In case of herbicides data on effect on soil physico-chemical and biological properties, and effect on normally cultivated three succeeding crops is required along with residue studies in the same plots of the field. Example: For a herbicide intended to be registered for use in wheat crop data on effect on succeeding crops of mRze at location 1, green gram at location 2, sesamum at location 3, may be generated along with residue studies. However, this is only an example and data on any other normally cultivated succeeding crop may be generated.) (ii) For the requirement of data on translocation in plants, International data from any authentic source shall be accepted. (iii) R+ : In case of TIM Vs TI and TI Vs TIM under section 9(3), one season data on bioefficacy including phytotoxicity, if any, on two representative crops at two climatic zones is required to be submitted. (iv) R^ : One season residue data on two representative crops particularly on fruits 4 and vegetables is required in case of TIM Vs TI and TI Vs TIM under section 9(3). (V) R++: In case of TI (New Source) two seasons data on each crop mentioned in labels/ leaflets at least at two Climatic Zones required on bio-effectiveness and phytotoxicity and two years or seasons data on Residues in plant on representative crops of each group on which pesticide is approved. NOTE: Data on Bioeffectiveness & Phyto-toxicity to be submitted on all registered formulations of same technical as per RC guidelines on all approved crops at the time of issue of import permit provided the application for registration is received within 4 years of issue of import Permit as per the decision of 313th meeting of RC held on 8th Nov, 2010. TIM*(AR) Vs TI U/s 9(4): If chemical equivalence fRls in the sample submitted by the applicant / inprocess samples, required to submit data on bioefficacy as per the guidelines for TIM vs TI U/s 9(3) C. TOXICITY 32. Acute oral in rat & mice R R R R R R R R R R R R 33. Acute dermal R R R R R R R R R R R R 34. Acute inhalation R R R R R R R R R R R NR 35. Primary skin irritation R R R R R R R R R R R R 36. Irritation to mucous membrane R R R R R R R R R R R R 37. Sub-acute oral rat R R NR/R R R NR/R NR/R NR NR R NR/ R NR 38. Sub-acute oral dog R* R* NR/ R* R* NR/ R* NR NR R* NR/ R* NR 39. Sub-acute dermal R R NR/R R R NR/R NR/R NR NR R NR/ R NR 40. Sub-acute inhalation R R NR/R R R NR/R NR/R NR NR R NR/ R NR 41. Neuro-toxicity NR NR NR R R NR/R NR/R NR NR NR NR/ R NR 42. Synergism & potentiation NR NR NR R R NR/R NR/R NR NR NR NR NR 43. Teratogenicity NR NR NR R R NR NR NR NR NR NR NR 44. Effect on reproduction NR NR NR R R NR NR NR NR NR NR NR 45. Carcinogenicity NR NR NR R R NR NR NR NR NR NR NR 46. Metabolism NR NR NR R R NR NR NR NR NR NR NR 47. Mutagenicity NR NR NR R R NR NR NR/R NR/R R NR NR 48. Toxicity to birds (two) R R R R R R R NR NR R NR NR 49. Toxicity to fish (fresh water) R R R R R R R NR NR R NR NR 50. Toxicity to honeybees R R R R R R R NR NR R NR NR R* NR/ R* 5 51. Toxicity to live stock R R NR R R NR NR NR NR R NR NR 52. Medical data R R R R R R R R R R NR R 53. Human toxicity R information from foreign countries R R R NR NR NR NR NR R NR NR 54. Observation in man NR (Health records of spray operators) NR NR NR NR NR^^ NR^^ NR NR NR NR^^ NR 55. Health records of Industrial workers. NR NR NR R NR R NR R NR NR 56. Toxicity to live stock (Field trial & observation) NR NR NR NR NR NR^^ NR^^ NR NR NR NR NR 57. International report on carcinogenicity & genotoxicity status NR NR NR R/NR R/NR NR NR NR NR NR NR NR R NR NR^^: Not required as per decision of 318th RC held on 27-04-2011 NF** : In case of wettable powder, if toxicological data is generated for EC formulation applicable as per guidelines, then there is no need to generate data on wettable powder contRning the same a.i. TIM*(AR) Vs TI U/s 9(4): If chemical equivalence fRls in the sample submitted by the applicant / inprocess samples, required to submit data on toxicity as per the guidelines for TIM vs TI U/s 9(3) R*: Any peer reviewed published data/information shall be acceptable (approved in 343rd and 344th RC Meeting) D. PACKAGING 58. Labels and leaflets as R per IR-1971 existing norms (i) for size 250 ml & below (ii) for 500 & above. R R R R R R R R R R R 59 Labels to contents R R R R R R R R R R R R a. DetRled Chemical composition R R R R R R R R R R R R b. Purpose for import / manufacture. R R R R R R R R R R R R c. Antidote R R R R R R R R R R R R d. Toxicity triangle R R R R R R R R R R R R e. Cautionary statement R R R R R R R R R R R R f. Brief direction concerning usages R R R R R R R R R R R R g. Restriction if any R R R R R R R R R R R R 60. Leaflets to contRn a. DetRled Chemical composition on leaflets NR NR R NR NR NR R R R NR R NR 6 accompanying small labels (upto 250 ml size contRner) b. Introductory para about the pesticide R R R R R R R R R R R R c. DetRled directions concerning usages NR NR R NR NR R R NR NR NR R NR d. Time of application NR NR R NR NR R R NR NR NR R NR e. Application equipment NR NR R NR NR R R NR NR NR R NR f. WRting Period NR NR R NR NR R R NR NR NR R NR g. Symptoms of poisoning R R R R R R R R R R R R h. First Rd measures R R R R R R R R R R R R i. Antidote & treatment R R R R R R R R R R R R j. Restriction, if any R R R R R R R R R R R R k. Instruction for storage R R R R R R R R R R R R l. Information regarding disposal of used packages. R R R R R R R R R R R R 61. Type of packaging (pkg R material + compatibility with content) R R R R R R R R R R NR 62. Manner of packaging R R R R R R R R R R R R 62.1 Specification for primary package R R R R R R R R R R R R 62.2 Specification for secondary packaging. R R R R R R R R R R R R 62.3 Specification for transport packaging. R R R R R R R R R R R R 63. Manner of labelling NR R R NR R NR R R R R R R 64. Performance of NR contRner during storage stability test NR NR R R R R R R R R NR 65 Transport worthiness test NR NR R R R R R R R R R NR 7 8