Experiment 8
advertisement

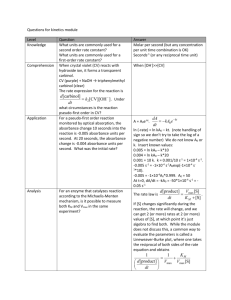
Sulatanat of Oman Sultan Qaboos university Collage of science Department of Chemistry CHEM4441 Experiment 8 : Simultaneous Spectrophotometric Determination Done by: lora ID #: Date: 30/4/2011 1 Objectives: To determine simultaneously the concentration of potassium permanganate and potassium dichromate in an aqueous solution by measurement of the absorbance of the solution at to wavelengths equal to 440 and 525nm. Introduction: Spectroscopy is the analysis of a substance by the measurement of the omitted light. The intensity of the light omitted is proportional to the concentration of the analyzed sample. In this experiment Potassium permanganate and Potassium dichromate were obtained and both adsorb visible light. Potassium permanganate KMnO4 is also known as "permanganate of potash". The permanganate ion is a strong oxidizing agent. It dissolves in water to give deep purple solutions. Potassium dichromate, K2Cr2O7, is a common inorganic chemical reagent, most commonly used as an oxidizing agent in various laboratory and industrial applications. It is potentially harmful to health and must be handled and disposed of appropriately. It is a crystalline ionic solid with a bright, red-orange color. The absorbance of the solution as we know at any wavelength is the sum of the absorbance of all the species in the solution. So, it is equal to: A x b[ X ] Y b[Y ] Z b[Z ] ............ Where… -A is the absorbance which you will measure with an instrument. 2 -ε is the molar absorptivity which represents how much light will be absorbed by 1 cm of a dilute solution of this chemical with units of L mol-1 cm-1. - b is path length measured in centimeters(usually 1cm). - [X], [Y],.. is the concentration measured in mol L-1. In this experiment we will analyze a mixture of two compounds, by measuring the absorbance at two wavelengths and knowing ε at each wavelength for each compound. Also, the absorbance of the mixture at any wavelength will be the sum of each component at that wavelength. The absorbance of the mixture at wave lengths 440 and 525 are given by the expressions : A440total 440 Mnb[Mn] 440Cr b[Cr ] and A525total 525Mnb[Mn] 525Cr b[Cr] The absorbance will be determined experimentally and then we will find the absorptivity constant witch is (ε b) from the equation of the graph that we will plot. This graph will represent the concentration vs. absorbance at certain wavelength and for certain compounds. And the concentrations of [X] and [Y] will be measured as: 3 'Y b A' ' ' 'Y b [X ] ' x b 'Y b ' ' x b ' 'Y b A' 'x b A' ''x b A' ' [Y ] ' x b 'Y b ' ' x b ' 'Y b In this experiment we have noticed that KMnO4 solution has a high absorption at 525 nm and lower at 440 nm. And K2Cr2O7 solution has a high absorption 440 nm and lower 525 nm. Experimental: Chemicals and equipments: Potassium permanganate Potassium dichromate H2SO4 solution 50ml volumetric flasks Pipettes. Spectrophotometer instrument. Procedure: First we are provided with standard solutions which are: solution (A) of KMnO4 (5.0× 10-4M). Also solution (X) ( 1.0 x 10-3 M ). Then 8 standards solutions(B,C,Y,Z,G,F,I and J ) were prepared from these two solutions to give different concentrations as this table show. 4 Solution Volume of (A) added (mL) Volume of (X) added (mL) B 5.0 0.0 C 10.0 0.0 Y 0.0 5.0 Z 0.0 10.0 G 5.0 10.0 F 5.0 10.0 I 10.0 5.0 J 10.0 5.0 Table#1: Dilute solutions of pure and mixed of KMnO4 and K2Cr2O7 Then, we measure the absorbance of pure KMnO4 solutions (A-C) and pure K2Cr2O7 solutions(X-Z) and the mixtures (G-J) at 440nm first and then at 525nm. At the end we measure the absorbance of our unknown at both wavelengths. Results: Unknown# 42 Sample Concentration (M) Absorbance (nm) at 440 nm Absorbance (nm) at 525 nm 5.00×10-4 0.098 0.532 B 1.00×10-4 0.029 0.163 C 2.00×10-4 0.052 0.301 X 1.00×10-3 0.191 0.058 Y 2.00×10-4 0.044 0.016 Z 4.00×10-4 0.087 0.030 G 6.65x10-4 0.118 0.193 F 5.035x10-3 0.119 0.189 A 5 I 5.71x10-4 0.099 0.313 J 5.59x10-4 0.097 0.302 blank 0.5 0.00 0.00 unknown 7.36x10-3 0.188 0.327 6 ε440Mnb = 168.08M-1cm-1 ε440Crb = 887.31M-1cm-1 ε525Mnb = 181.351M-1cm-1 ε525Crb = 51.154 M-1cm-1 Calculations: See the appendix 7 Discussion & answering Question: -The main sources of errors in this experiment are: Pipetting the correct volume of the solutions. Standardization errors . Indeterminate errors of spectrophotometer instrument. Fingerprintes on the spectrophotometer cell. -Graphs obtained in the calculation part have linearity of above 99 % which is good. 1- absorbance is additive so, the absorbance of the mixture will be the sum of the absorbance for both X and Y at each wavelength (λa and λb).Obtaining the concentration for both X and Y will help to get the value of the ε for each . The concentrations of both X and Y in the mixture could be determined. 2- No, because the absorbance band will be overlapped everywhere . The appropriate method is the least-squares analysis by a spreadsheet. Because this method will give more accurate values for the concentrations of the compounds in the mixture. Observations: The K2Cr2O7 solution is yellow. The KMnO4 solution is violet. The mixture of K2Cr2O7 and KMnO4 is pink. 8 The KMnO4 solution has high absorbance at 525 nm and low one at 440nm. The K2Cr2O7 solution has high absorbance at 440 nm and low one at 525nm. A+X and B+X solutions have high absorbance at 440 nm and lower one at 525nm. Conclusion: We are achieved all the objectives of this experiment and obtained: Concentration of KMnO4 in unknown = 0.0003 M Concentration of K2Cr2O7 in unknown = 0.000706 M References: The experimental handout. Daniel C. Harris, Quantitative Chemical analysis , 6th edition, Published by Michelle Russel Julet, 1982, Freeman and Company, New York – Ch19. 9