PHY 3513: Spring 2006 Final Exam Name:_________________________
advertisement

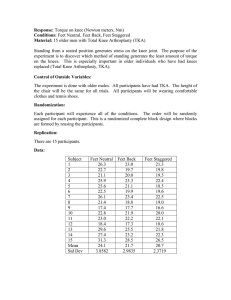
PHY 3513: Spring 2006 Final Exam Name:_________________________ 1. Consider the Joule cycle heat engine consisting of two isobars (at pressures P1 and P2)and two adiabats. Assuming that the working substance is an ideal gas with the constant specific heat capacities, Cp and Cv = Cp/γ, show that the efficiency of this engine can be written as ⎛ 1⎞ ⎜ 1− ⎟⎟ γ⎠ ⎛ P ⎞ ⎜⎝ η = 1 − ⎜⎜ 1 ⎟⎟ ⎝ P2 ⎠ 2. Suppose that the volume thermal expansivity β = (v-a)/vT and κ = 3(v-a)/(4Pv). Show that the equation of state is given by P3/4(v-a) = AT Where a and A are constants. 3. (a) If Cv = a(P)T + b(P)T3 at low temperatures (as is the case with most metals, ask Stewart or Andraka), calculate the temperature variation of the entropy. (b) Calculate the temperature dependence of the coefficient of volume thermal expansion (vβ = dv/dT|P) from the entropy in part (b). If β>o, what does that say about the coefficients a(P) and b (P).