Lesson 6 -- Vapor Pressure
advertisement

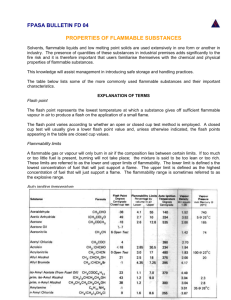
Transcona Community Learning Centre CHEM 30S LESSON 6: VAPOR PRESSURE We have learned about the production of vapour during evaporation and boiling. In this lesson, we will study the relationship between the pressure produced by this vapour and the boiling point of a substance. Lesson Outcomes When you have completed this lesson, you will be able to: Operationally define vapour pressure in terms of observable and measurable properties. Construct and interpret a graph of vapour pressure versus temperature. Interpolate and extrapolate vapour pressures of substances at various temperatures from vapour versus temperature graphs. Describe vaporization at the molecular level using diagrams. Explain why vapour pressure varies with temperature, citing examples (e.g., explain why Canadian homes are ‘drier’ in the winter than in the summer). Describe the practical value of knowing the vapour pressures of various substances. Operationally define normal boiling temperature and boiling temperature in terms of vapour pressure. Pressure According to the Kinetic Molecular Theory, the particles of a gas are constantly moving in random straight-line motion. If the gas particles are in a container, the particles must eventually collide with the sides of the container or other gas particles. When the particles collide with the sides of the container, they exert a force upon the container’s walls. We call this force gas pressure. Pressure is defined as force per unit area. The Earth's atmosphere is about 100 km thick. This means each object on or above the Earth has a column of air (the atmosphere) pushing down on it. The force created by this column of air is called air pressure or atmospheric pressure. Units of Pressure Units of pressure are often related to units of force. The kiloPascal (kPa) is a Newton of pressure per square metre of area. The kPa is the SI unit of pressure. Aug 2006 Module 1 32 Transcona Community Learning Centre CHEM 30S Another unit of pressure is the millibar. The millibar is a meteorological unit of atmospheric pressure. One bar is equal to standard atmospheric pressure or 1 atmosphere. The unit “atmosphere (atm)” was derived from standard atmospheric pressure at sea level. 1 atmosphere is equal to 760 mmHg, or 101.325 kPa. Two atmospheres is twice standard atmospheric pressure, and so on. Mm of mercury is not a common unit used today outside the laboratory, however, many aneroid barometers found in the home use both mm of mercury as well as another unit like kilopascals. We will discuss the historical development of these units further in the next module. Measuring Pressure Pressure can be measured using several devices or instruments. A manometer usually has a bulb or glass container on one end and can be open or closed on the other. A liquid, often mercury, is placed in a U-shaped tube. The pressure is measured by finding the difference in height on both sides of the tube. An example of a manometer is shown below. Aug 2006 Module 1 33 Transcona Community Learning Centre CHEM 30S The greater the pressure of the gas in the bulb, the greater the force on the mercury and the higher the level of the mercury on the right side of the U-shaped tube. We will discuss measuring gas pressure to much greater extent in the next module. Behaviour of a Liquid in a Closed Container Liquid left in an open container will eventually evaporate to dryness. The particles on the surface of the liquid vaporize and leave the container. If the container is sealed, the vapour is trapped and cannot leave the container. Eventually, the space above the liquid becomes saturated with vapour and for every molecule that evaporates, another condenses. Liquid in a closed container, at a constant temperature, reaches equilibrium with its vapour. At equilibrium, rate of evaporation = rate of condensation The pressure created by the vapour at equilibrium is known as the vapour pressure, abbreviated Pvap. Vapour pressure is a characteristic physical property because many substances have different vapour pressures. Vapour Pressure and Intermolecular Forces Intermolecular forces determine the rate of evaporation. Try putting a few drops of rubbing alcohol and water on the countertop. Which evaporates faster? The alcohol should disappear more quickly. The temperature of both liquids is the same (if both at room temperature), so their intermolecular forces must determine which evaporates faster. The alcohol has lower intermolecular forces than the water, so it evaporates faster. Aug 2006 Module 1 34 Transcona Community Learning Centre CHEM 30S If the intermolecular forces are lower, the amount of vapour that accumulates increases because it is easier for the particles to escape the forces of attraction in the liquid and become gaseous. The manometers above show the difference in the height of the mercury to be much greater with alcohol in the vessel, as compared to water in the vessel. Generally, the lower the forces of attraction, the higher the vapour pressure. Substances that evaporate easily, such as alcohol and acetone, are said to be volatile. The lower the forces of attraction, the greater the volatility. Often, volatile substances are also very flammable. Vapour Pressure and Temperature There also exists a relationship between temperature and vapour pressure for a substance. As temperature increases, the amount of vapour also increases. The rising temperature causes more particles to have enough energy to overcome the forces of attraction of the liquid. This increases the rate of evaporation, increasing the number of vapour particles.. If the number of vapour particles increases, the pressure increases. Aug 2006 Module 1 35 Transcona Community Learning Centre CHEM 30S Vapour Pressure and Boiling Point In an earlier lesson we described boiling in terms of when vaporization occurs throughout the liquid and the temperature of the liquid remains constant. This is known as an operational definition of boiling. The boiling point of a substance is defined as the temperature at which the vapour pressure of a substance is equal to the atmospheric pressure. Let's examine how this works. As the temperature of a liquid increases, the vapour pressure also increases. Bubbles of vapour begin to form in the liquid and rise towards the surface. Once the pressure inside the bubble is great enough to overcome the atmospheric pressure, the bubble is able to release the vapour it contains. If the atmospheric pressure is greater than the vapour pressure, the bubble will be kept below the surface until the pressure increases. Atmospheric Pressure and Boiling Point We have already determined that intermolecular forces affect boiling point. The greater the intermolecular forces, the lower the vapour pressure and the greater amount of energy needed to produce enough vapour, so that vapour pressure equals atmospheric pressure. Aug 2006 Module 1 36 Transcona Community Learning Centre CHEM 30S Since boiling occurs when atmospheric pressure and vapour pressure are equal, any change in pressure will produce a change in the boiling point. The normal boiling point of a substance is defined as the temperature when vapour pressure equals standard pressure (1 atmosphere, 101.3 kPa, 760 mmHg, 760 torr). The lower the atmospheric pressure, the lower the boiling point. Since less vapour pressure is required, less heat is needed. This explains why it requires longer cooking time to prepare foods at higher altitudes. At higher altitudes, the atmospheric pressure is lower because the column of air is shorter. The lower the air pressure, the lower the boiling point. If we were to try to hard boil an egg at a high altitude, the water would boil at a much lower temperature. The egg would take much longer to cook. See the table below. Altitude Boiling Point of Water Sea Level 100°C 2000 feet 98°C 5000 feet 95°C 7500 feet 92°C 10 000 feet 90°C Pressure cookers are often used to speed up cooking. A pressure cooker operates by increasing the pressure inside the pot. As pressure increases, the boiling point of the water also increases because more energy is needed to create more vapour. The higher the temperature at which water boils, the shorter the cooking time. Vapour Pressure Curves We can plot the vapour pressure as a function of temperature and get a graph like the one that follows. You will notice that regardless of the substance there is a similar trend in the vapour pressure as temperature increases. Aug 2006 Module 1 37 Transcona Community Learning Centre CHEM 30S We can use this graph to determine the normal boiling point of each substance and the temperature each will boil at various pressures. Example 1 What is the normal boiling point of ethanol? Solution From the graph, we look for the temperature at which the vapour pressure of ethanol is 101.3 kPa (standard pressure). According to the graph, the normal boiling point is about 77°C. Example 2 If the pressure in a city is 90 kPa, at what temperature will water boil? Solution When the atmospheric pressure is 90 kPa, water will boil when its vapour pressure is 90kPa. We start at 90 kPa on the y-axis and move to where it intersects with the curve for water, then see to what temperature this Aug 2006 Module 1 38 Transcona Community Learning Centre CHEM 30S corresponds. According to the graph, water boils at about 97°C at 90 kPa of pressure. Example 3 Which substance has the largest forces of attraction at 60°C? Solution The substance with the largest forces of attraction, will have the lowest vapour pressure. The substance with lowest vapour pressure at 60°C is acetic acid. Lesson Summary In this lesson, you have learned: A barometer is used to measure atmospheric or air pressure and a manometer is used to measure gas pressure. Gas pressure is the force of gas particles on the sides of their container. Gas pressure is directly proportional to the temperature and the number of particles in the container. Vapour pressure is the pressure produced by a vapour in a closed container in equilibrium with its liquid phase. Vapour pressure increases with increased temperature. The lower the intermolecular forces, the higher the vapour pressure. The boiling point of a substance is the temperature at which the vapour pressure equals the air pressure. The normal boiling point is the temperature at which the vapour pressure of the liquid is equal to standard pressure. Assignment Use the vapor pressure curves on Page 38 to answer the questions below: 1. 2. 3. 4. 5. 6. 7. 8. 9. Aug 2006 What is the vapour pressure of ethanol at 60°C ? What is the vapour pressure of acetic acid at 80°C ? What is the approximate vapour pressure of chloroform at 0°C? What is the temperature at which the vapour pressure of ethanol is 50 kPa? Which of these substances has the highest vapour pressure? Which of the three substances would evaporate fastest at room temperature? Which of the three substances would evaporate slowest at room temperature? Which substance has the weakest intermolecular forces? From the graph, what are the normal boiling points of the four substances? Module 1 39 Transcona Community Learning Centre CHEM 30S 10. What would be the boiling point of water on a day when the atmospheric pressure is 95 kPa? 11. Alcohol is heated in a container in which there is a partial vacuum. The air pressure in the container is 25 kPa. At what temperature will the alcohol boil? 12. If substance “X” had a normal boiling point of 30°C, where would you expect to find the vapour pressure curve of “X”? Explain your answer. i) to the left of the chloroform curve? ii) between the chloroform and ethanol curve? iii) between the ethanol and water curve? iv) between the water and acetic acid curve? v) to the right of the acetic acid curve? 13. In the definition of vapour pressure, there are the words “in a closed container”. Why is it necessary to have a closed container? 14. What would the atmospheric pressure have to be in order to have ethanol boil at 20.0°C ? 15. What would the atmospheric pressure be to have acetic acid boil at 80.0°C ? 16. If the temperature was 50.0°C and the atmospheric pressure was 20 kPa, which substances if any would boil? 17. If the temperature was 80.0°C and the atmospheric pressure was 100 kPa, which substances if any would boil? Aug 2006 Module 1 40