CH437 CLASS 12
advertisement

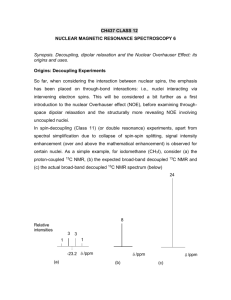
CH437 CLASS 12 NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY 6 Synopsis. Decoupling, dipolar relaxation and the Nuclear Overhauser Effect: its origins and uses. The Nuclear Overhauser Effect Origins: Decoupling Experiments So far, when considering the interaction between nuclear spins, the emphasis has been placed on through-bond interactions: i.e., nuclei interacting via intervening electron spins. This will be considered a bit further as a first introduction to the nuclear Overhauser effect (NOE), before examining throughspace dipolar relaxation and the structurally more revealing NOE involving uncoupled nuclei. In spin-decoupling (Class 11) (or double resonance) experiments, apart from spectral simplification due to collapse of spin-spin splitting, signal intensity enhancement (over and above the mathematical enhancement) is observed for certain nuclei. As a simple example, for iodomethane (CH3I), consider (a) the proton-coupled 13C NMR, (b) the expected broad-band decoupled 13C NMR and (c) the actual broad-band decoupled 13C NMR spectrum (below) 24 8 Relative intensities 3 1 3 1 -23.2 /ppm (a) /ppm (b) /ppm (c) 1 Suppose T1CC is T1 relaxation due to interactions between carbon-13 nuclei. T1HH is T1 relaxation due to interactions between hydrogen nuclei. T1CH is T1 relaxation due to interactions between due to 13C 13C and hydrogen nuclei. MZ(C) is the magnetization nuclei. Mo(C) is the equilibrium magnetization of 13C. MZ(H) is the magnetization due to hydrogen nuclei. Mo(H) is the equilibrium magnetization of hydrogen. The equations governing the change in the z magnetization with time are: If we saturate the proton spins, MZ(H) = 0. Letting the system equilibrate, d MZ(C) /dt = 0. Rearranging the previous equation, we obtain an equation for MZ(C): 2 . Note that MZ(C) has increased by Mo(H) T1CC / T1CH which is approximately 2 Mo(C), giving a total increase of a factor of 3 relative to the total area of the undecoupled peaks. This explains the extra factor of three (for a total intensity increase of 24) for the carbon-13 peak when hydrogen decoupling is used in the 13C NMR spectrum of CH3I. Origins of Dipolar Relaxation and the (Through Space) Nuclear Overhauser Effect Now consider two uncoupled protons in the same molecule. To give an NMR signal, excess spin population has to be moved from one energy level to another (excitation), followed by radiationless return to equilibrium (spin-lattice relaxation). The excess energy passes from spins to the lattice (the environment or surroundings) as heat, but for efficient relaxation, fluctuating magnetic fields of the right frequency are needed. In most molecules, these fields are produced by the magnetic moments of other protons in the same molecule, as they tumble (along with the rest of the molecule) in solution. This dipole-dipole interaction is the basis of dipolar relaxation. Dipolar relaxation efficiency (or rate) depends on several factors, as outlined in the branch diagram below. 3 Internuclear distance Dipolar relaxation rate Effective correlation time (tc) of the vector that joins the two nuclei (= the reciprocal of the rate of tumbling of that part of the molecule) Strength and frequency of fluctuating magnetic fields Identity of the two nuclei In particular, NOE and the rates at which they grow and decay are measures of the strength the dipole interaction between the nuclear spins and hence are dependent upon internuclear distance and correlation times. It is these aspects that give NOE its great value as a structural probe More detailed consideration of two uncoupled nuclei Ha and Hx is considered in the diagram below. The use of symbols a and x should be confused with symbols A and X in coupled spin systems. Aligned Opposed Ha Hx Hx Ha Ha Bo Tumbling motion ~ 108 s-1 for large molecules and ~ 1011 s-1 for small molecules. (Assume a nearly spherical molecule, which tumbles isotropically) Hx Bo Tumbling The fluctuating magnetic field at Hx is caused by change in alignment of Ha with the applied field Bo, upon change in relative position during tumbling 4 The dipolar relaxation of Hx is more efficient in more slowly rotating (tumbling) systems and when Ha and Hx are close: for NOE observation, r(Ha – Hx) < 0.3 nm (300 pm). The origin of NOE may be understood by considering the energy transition diagram for an uncoupled two-spin system (say, Ha and Hx, as before), below. + 1 _ W1x W1a W2 3 2 W0 E W1x _ + W1a 4 xa Transitions W 1a correspond to the inversion of spin Ha and W 1x to inversion of spin Hx. Since Ha and Hx are shielded by their surrounding electrons to different extents (they are in different magnetic environments), the W 1a and W1x transitions involve the absorption of different amounts of energy, such that (4) (2) = (3) (1) and (4) (3) = (2) (1). Hence, in the absence of spin-spin coupling, there is one resonance line for each nucleus. The transitions W 0 and W 2 are forbidden by direct absorption or emission of radiation, because they involve changing the spins of both nuclei simultaneously, but are very effective radiationless relaxation transitions. Under normal thermal equilibrium conditions, the lower energy levels are slightly more highly populated than the upper levels. If Ha is strongly irradiated by the decoupling frequency, saturation occurs, such that (1) undergoes a population increase (+), whereas (3) experiences population decline (-). Similarly, (2) is increased in population by +, whilst (4) is decreased in population by -. Now, the intensity of signal of the observed nucleus (Hx) depends on the extent to 5 which the sum of the population levels (1) and (3) is less than the sum of levels (2) and (4). The effect of irradiating Ha does not effect the intensity of the signal for Hx via the W 1x transitions, since both (4) and (3) have been depleted by and both (2) and (1) have been increased by . However, W 0 and W 2 are nonradiative transitions (and are both forbidden for radiative transitions): they represent dipolar relaxation mechanisms. W 0 is small and so corresponds to slower tumbling motions of large molecules, hence for these molecules, the population differences (+ and -) produced by irradiation of Ha will lead to more (2) (3) transitions than in the absence of radiation. This results in an increase of the sum of the populations of levels (1) and (3): it nearly approaches the sum for levels (2) and (4). As the difference between these sums determines the intensity of the signal from Hx, the effect of radiating Ha is to decrease this intensity: this is the negative NOE. On the other hand, W 2 is large and corresponds to the faster tumbling of smaller molecules, leading to more (1) (4) transitions upon irradiation of Ha. This causes the lower energy levels of Hx to have higher populations than the higher energy levels, so that the effect of radiating Ha is to increase the intensity of the Hx signal: this is the positive NOE. General Comments on NOE Signal enhancement due to NOE is an example of cross-polarization: a throughspace effect in which polarization of spin states of one type of nucleus (say 1H) caused by irradiation (B1) induces polarization of spin states of another nucleus (say, 13C or 1H). In general, the maximum NOE enhancement is given by NOEmax = 1 2 irr obs Here, irr is the magnetogyric ratio of the nucleus being irradiated and obs is that for the nucleus under observation. The total (maximum) line intensity is given by 1 + NOEmax. Hence, in the case of proton-decoupled 13C NMR spectra, the 13C 6 signal can be enhanced up to 200% by the irradiation of protons. This value is a theoretical maximum: most actual 13C lines exhibit less (sometimes much less) than maximum enhancement. Uses of NOE 1. In Proton-Decoupled 13 C Spectra In a proton-decoupled 13C spectrum, the total NOE for a given 13C nucleus increases as the number of nearby protons increases. Hence, the intensities of signals in a 13C spectrum (assuming a single carbon of each type) are usually in the order C < CH < CH2 < CH3. This is often reliable for distinguishing unprotonated carbon atoms from protonated ones, but otherwise can be unreliable, as shown in the spectrum below. For more reliable criteria regarding numbers of protons bonded to carbon atoms, see J-scaling (Class 11), DEPT (Class 15) and COSY (Class 16). 2. Determination of Stereochemical Relationship Between Nuclei Because NOE is a through-space effect, observed nuclei at some distance from the nucleus under irradiation suffer smaller (positive or negative) intensity changes. In fact, magnitude of the NOE falls off as a function of the inverse of r 3: 7 r 13 C 1 H NOE = f 1 r3 This can be exploited in the assignment of diastereotopic protons and in the determination of molecular stereochemistry. An example of the former is given below and after that there is an example of the latter. Example 1. Dimethylformamide .. :O C .. N H CH3 _ .. : .. O CH3 C CH3 H + N CH3 The methyl groups are non-equivalent, because of the considerable double bond character of the C-N bond: their 13C nuclei resonate at 31.1 and 36.2 ppm: but which is syn and which is anti (with respect to the H atom)? Irradiation at the 1H frequency of the aldehyde group leads to a greater Nuclear Overhauser enhancement of the signal at 36.2 ppm. This must be the one spatially nearer the aldehyde proton, so the assignment is .. :O C .. N H CH3 31.1 ppm CH3 36.2 ppm Example 2. Isovanillin OCH3 HO Ha Hc Hb CHO In the 1H NMR spectrum of isovanillin, there are three aromatic ring protons and although it is possible to assign them according to chemical shift tables, 8 observation of NOE can be of additional help. If the sample is irradiated at the proton frequency of the OCH3 group, the intensity of the signal due to Ha (the nearest proton to OCH3) is enhanced. Example 3. The proximities of the protons indicated by double-headed arrows were established by observation of NOE from double resonance experiments. O H H O O O CH3 O CH3 H O H or CH3COO O CH3COO H CH3 O H CH3 O O Example 4. Configuration of a Synthetic Penicillin Analog Irradiation of the methyl protons shown causes enhancement of the signals due to H(10) and H(3), indicating that both of these are spatially close the methyl group: configuration A is the major one. H3C 10 H S H N H 3C CH 3 S S H N 3 CO2CHPh2 S O A 10 H CH 3 3 CO2CHPh2 O B Example 5. Sucrose Octa-acetate This example illustrates the use of NOE difference spectra 9 6 AcOCH2 O AcO 4 AcO 5 3 2 OAc 1' CH2 OAc O 1 OAc O 2' AcO 3' 5' 4' CH2OAc 6' Irradiation of the H2 (an axial proton) multiplet reveals large NOEs to the two nearest “cis” protons, H1(equatorial) and H4 (axial), with the “trans” H3 being only slightly affected, as seen in the NOE difference spectrum below. The large arrow indicates the frequency of irradiation. The pulse sequence for the simplest NOE difference experiment is shown below, along with the corresponding spectra and the difference spectrum. The spectrum derived from sequence (b) is subtracted from that arising from sequence (a). In (a), a low-power irradiating pulse of (180o) duration causes inversion of the zmagnetization of one particular nucleus, say spin 2. This is followed by a delay () and finally the z-magnetization is made observable by a non-selective /2 (90o) pulse. This gives the irradiated spectrum. The experiment (b) is simply a pulse-acquisition sequence that results in the normal spectrum. 10 Only spins that have received NOE enhancement appear in the difference spectrum (above, nucleus 1), along with the inverted signal of the irradiated spin (above, nucleus 2). The above assumes a positive cross-relaxation rate constant, so the NOE enhancement is positive. In practice, the simple pulse sequence for (a) shown above sometimes gives rather poor NOE difference spectra and so is replaced by an inversion sequence that uses pulsed field gradients. 11