chapter5sgfall09ans
advertisement

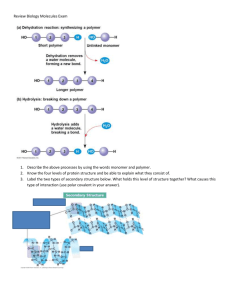
CHAPTER 5 The Structure and Function of Macromolecules The Principles of Polymers READ: Skim Overview and Section 5.1, carefully read “The Synthesis and Breakdown of Polymers” to answer questions. 1. List the four major classes of macromolecules. Carbohydrates Lipids Proteins Nucleic Acids 2. Distinguish between monomers and polymers. Monomer- mono = 1 subunit of a macromolecule Polymer- poly = many monomers linked together Monomers are repeating units that serve as the building blocks for a polymer 3. Draw diagrams to illustrate dehydration and hydrolysis reactions. (When is water lost, when is it used? See figure 5.2 Dehydration- joins monomers together to form polymers, water is lost Hydrolysis- breaks polymers apart into monomers, water is used Carbohydrates Serve as Fuel and Building Material READ: Skim Section 5.2 and carefully read text relating to Figures 5.5 and 5.7 to answer Questions. 4. Distinguish between monosaccharides, disaccharides, and polysaccharides. Monosaccharide = monomer of the carbohydrate macromolecule Disaccharide = 2 monomers linked together by a dehydration reaction Polysaccharide = 3 or more monomers linked together by dehydration reactions 5. Describe the formation of a glycosidic linkage. This is the covalent bond that forms between carbohydrate monomers linked together in a dehydration reaction. Also be able to….Identify the monomer of carbohydrates and the bond that links these monomers. Monomer: monosaccharide Bond: glycosidic 6. Distinguish between the glycosidic linkages found in starch and cellulose. Explain why the difference is biologically important. Starch- ALPHA glucose monomers are linked by glycosidic bonds. Your body CAN break the glycosidic linkages between these ALPHA glucose monomers. Cellulose- BETA glucose monomers are linked by glycosidic bonds. Your body CANNOT break the glycosidic linkages between these BETA glucose monomers. This question put another way …. Starch, like what you would find on the INSIDE of a baked potato, and Cellulose, what you would find in the potato’s skin, are both made of glucose monomers. What type of glucose monomer would you find in starch and what type of glucose monomer would you find in the cellulose? Distinguish between the glycosidic linkages found between alpha glucose monomers and those between beta glucose monomers. Explain why the difference is biologically important. Starch- ALPHA glucose monomers are linked by glycosidic bonds. Your body CAN break the glycosidic linkages between these ALPHA glucose monomers. If your body can break the linkage between the glucose monomers, you can absorb the glucose from the digestive tract and use it as an energy source. Cellulose- BETA glucose monomers are linked by glycosidic bonds. Your body CANNOT break the glycosidic linkages between these BETA glucose monomers. If your body cannot break the linkage between the glucose monomers, you cannot absorb the glucose from the digestive tract and cannot use it as an energy source. 7. Describe the role of symbiosis in cellulose digestion. Some animals, such as cows, have organisms in their intestines that CAN break the glycosidic linkages between BETA glucose monomers. Therefore the cow indirectly has the ability to break these beta linkages and use the beta glucose monomers to produce ATP. The organisms in the cow’s intestines also benefit, as they get a great place to live and food that the cow ingests. Lipids are a Diverse Group of Hydrophobic Molecules READ: Skim Section 5.3 and carefully read text relating to Figures 5.11, 5.12 and 5.13 to answer questions 8. Describe the building-block molecules, structure, and biological importance of fats, phospholipids, and steroids. This question put another way…. Compare and contrast the 3 major categories of LIPIDS: fat, phospholipids, and steroids by describing the: -building-block molecules (monomers) -structure -biological importance Fat: Triglyceride Building-block moleculesMonomers- glycerol and fatty acids Structure – 3 fatty acids linked to one glycerol Biological importanceEnergy source Phospholipids: Building-block moleculesMonomers- glycerol, fatty acids and a phosphate group Structure – 2 fatty acids and one phosphate group are linked to one glycerol molecule. The phosphate group is polar and the fatty acids part of the phospholipids is nonpolar. The Biological importance- Phospholipids are a major component of cell membranes and form a bilayer. The polar “head” is positioned toward the outside and inside of the cell, which has an affinity for the aqueous environment found both outside and inside the cell. The fatty acid tails of each layer of phospholipids are positioned toward the center of the membrane due to their nonpolar (water hating) nature. Steroids: Building-block molecules/structure4 carbon ring structure with various carbon side chains and functional groups linked to that 4 carbon ring structure. Biological importance- Several hormones are steroids, including estrogen and testosterone. 9. Identify an ester linkage and describe how it is formed. Just be able to identify what macromolecule’s (lipids) monomers are linked by ester bonds. The ester l forms by a dehydration reaction among the lipid monomers. Identify the linkage between the monomers of Lipids and describe how it is formed. Ester bonds. The ester linkage forms by a dehydration reaction among the lipid monomers. 10. Distinguish between saturated and unsaturated fats. Saturated = carbon skeleton is saturated with hydrogen atoms, NO double bonds so there is a very close association among lipid molecules. This is why saturated fats are solid at room temperature Unsaturated = carbon skeleton is NOT saturated with hydrogen atoms; double bonds are present in these molecules so there is not a very close association among these lipid molecules. This is why unsaturated fats are liquid at room temperature 11. Name the principal energy storage molecules of plants and animals. triglycerides Proteins have Many Structures and Many Functions READ: Skim Section 5.4 and carefully read text relating to Figures 5. 17, 5.18, 5.21 to answer questions 12. Distinguish between a protein and a polypeptide. Polypeptide- a chain of amino acids linked by peptide bonds Protein- a polypeptide chain or several polypeptide chains folded into the correct conformation also be able to… A. Identify the monomer of protein and the bond that links these monomers. B. Distinguish between a protein and a polypeptide. A. Monomer: amino acids, Bond: peptide bond B. Polypeptide- a chain of amino acids linked by peptide bonds Protein- a polypeptide chain or several polypeptide chains folded into the correct conformation 13. Explain how a peptide bond forms between two amino acids. Dehydration reaction: The amino group of one amino acid monomer is linked through dehydration to the carboxyl group of another amino acid monomer. 14A. List and describe the four major components of an amino acid. Explain how amino acids may be grouped according to the physical and chemical properties of the R group. Major components: 1. amino group 2. carboxyl group 3. R group (can be polar, nonpolar, electrically charged) 3 Rules for determining whether an amino acid is nonpolar, polar or electrically charged: 1. Does the R group have only carbon (C) and Hydrogen (H)? If yes, it is NONPOLAR! If no, move on to question 2. 2. Is the atom that is not C or H on the end of the R group or surrounded by carbon and hydrogen? If the atom that is not C or H is surrounded by C and H, the R group is NONPOLAR. If the atom that is not C or H is NOT surrounded by C and H, move on to question 3. 3. Is there a charge negative (-) or positive (+) on the atom that is not C or H? If no, then the R group is POLAR. If yes, then the R group is ELECTRICALLY CHARGED. 14B. The R group of the amino acid Serine is –CH2-OH. The R group of the amino acid Alanine is –CH3. Where would you expect to find these amino acids in a folded protein that is in and aqueous (water) solution? (HINT: Think of the 3 categories of amino acid side chains, how are Serine and Alanine categorized? How would this affect where they would be found in the protein?) Alanine would be in the interior and Serine would be on the outside of the protein. Alanine is a nonpolar amino acid, so it is also hydrophobic, water hating, It would hide from the water enviroment of a living thing by tucking itself inside the protein. Serine is a polar amino acid, so it is hy water loving. It likes substances that are like itself and would be just fine exposed to the water environ living thing and would be on the outside of the protein. 15. Explain what determines protein conformation and why it is important. Protein conformation (3-D structure) is in large part determined by hydrogen bonding among both the amino acid backbones of each amino acid in a polypeptide chain and between R groups. Other types of bonding occur between R groups that also contribute to the 3-D structure such as ionic bonding and disulfide bridges The 3-D structure is important because the structure of the protein determines its function. If the structure is lost the protein will not function. 16. Explain how the primary structure of a protein is determined. The order of the amino acids (primary structure) in a polypeptide chain is determined by the DNA sequence. 17. Name two types of secondary protein structure. Explain the role of hydrogen bonds in maintaining secondary structure. Alpha helices and beta-pleated sheet, result from hydrogen bonds between the backbones of amino acids in the polypeptide chain. The backbone of an amino acid includes the parts of the amino acid that is the same in every amino acid, the amino group the carboxyl group the hydrogen atom, everything EXCEPT the R group!!! 18. Bonds between which part of the amino acids contribute to tertiary protein structure? R groups Covalent bonding, ionic bonding, hydrogen bonding, disulfide bridges 19. List two conditions under which proteins may be denatured. Heat and pH change 20. What is a Chaperonin? A protein that assists in the folding of other protein molecules (polypeptideprotein) Nucleic Acids Store and Transmit Hereditary Information READ: Skim Section 5.5, carefully read text relating to Figures 5. 27 & 5.28 to answer questions 21. List the major components of a nucleotide, and describe how these monomers are linked to form a nucleic acid. Monomer: nucleotide Sugar, phosphate, nitrogenous base Bond: Phosphodiester bond KNOW YOUR CARBONS (1’-5’) ON THE SUGAR RING! Phosphate is linked to sugar at the 3’ and another at the 5’carbon of the sugar and the nitrogenous base is linked to the sugar at the 1’ carbon 22. Distinguish between: a. pyrimidine- C, T or U and purine- A or G b. nucleotide- phosphate, sugar and nitrogenous base and nucleoside- just the sugar and nitrogenous base c. ribose- sugar found in RNA polynucleotide and deoxyribose- sugar found in DNA polynucleotide d. 5’ end- 5’ carbon of sugar, place where phosphate is linked to sugar and 3’ end of a nucleotide 3’ carbon of sugar, place where phosphate of another nucleotide adds to the original nucleotide. 23. Briefly describe the three-dimensional structure of DNA. Two polynucleotide chains wrapped around one another to form a double helix 24. List at least 3 main differences between DNA and RNA. DNA-double stranded RNA- single stranded DNA- ATCG nuclotides RNA- AUCG nucleotides THIS IS IMPORTANT!!! DNA- deoxyribose sugar (no OH group on 2’ carbon of the sugar ring) RNA- ribose sugar (OH group IS on 2’ carbon of the sugar ring) Suggested end of chapter SELF-QUIZ questions: #1, 3, 4-9 See textbook appendix for answers