Animal Ethics Application form
advertisement
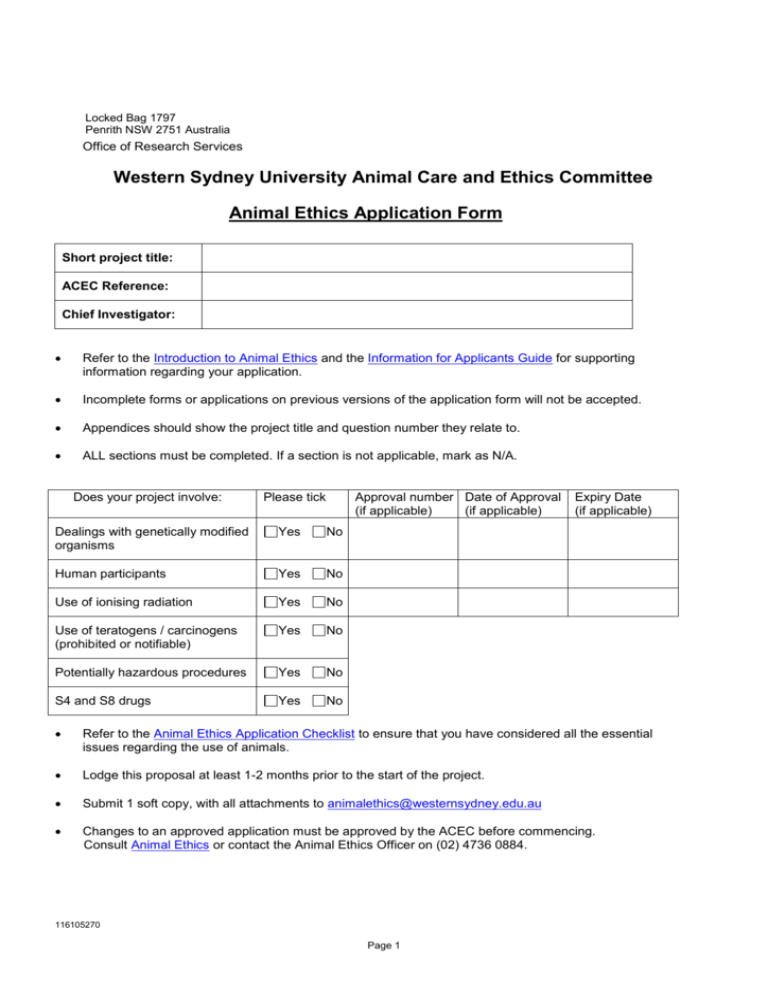
Locked Bag 1797 Penrith NSW 2751 Australia Office of Research Services Western Sydney University Animal Care and Ethics Committee Animal Ethics Application Form Short project title: ACEC Reference: Chief Investigator: Refer to the Introduction to Animal Ethics and the Information for Applicants Guide for supporting information regarding your application. Incomplete forms or applications on previous versions of the application form will not be accepted. Appendices should show the project title and question number they relate to. ALL sections must be completed. If a section is not applicable, mark as N/A. Does your project involve: Please tick Approval number Date of Approval (if applicable) (if applicable) Dealings with genetically modified organisms Yes No Human participants Yes No Use of ionising radiation Yes No Use of teratogens / carcinogens (prohibited or notifiable) Yes No Potentially hazardous procedures Yes No S4 and S8 drugs Yes No Expiry Date (if applicable) Refer to the Animal Ethics Application Checklist to ensure that you have considered all the essential issues regarding the use of animals. Lodge this proposal at least 1-2 months prior to the start of the project. Submit 1 soft copy, with all attachments to animalethics@westernsydney.edu.au Changes to an approved application must be approved by the ACEC before commencing. Consult Animal Ethics or contact the Animal Ethics Officer on (02) 4736 0884. 116105270 Page 1 A. ADMINISTRATION 1. Short project title: 2. Chief Investigator Name of Chief Investigator: School/Institute name: Contact phone/fax, email address: After hours phone/fax: Address for correspondence: In signing I certify that all details given in this proposal are correct and I agree to ensure the project is carried out in accordance with the Animal Research Act 1985 and Regulation 2010, and the Australian code of practice for the care and use of animals for scientific purposes (NHMRC; CSIRO; AAC). I also certify that the qualifications and experience of personnel involved in the project are appropriate to the procedures to be performed. Signature: Date: 3. Others involved in the project. Name: Role: Student Investigator Investigator Professional Staff School/Institute name: Contact phone/fax, email address: After hours phone/fax: Address for correspondence: Declaration In signing I certify that I am aware of my responsibilities as set out in the Animal Research Act 1985 and Regulation 2010 and the Australian code of practice for the care and use of animals for scientific purposes. Signature: Date: 116105270 Page 2 Name: Role: Student Investigator Investigator Professional Staff School/Institute name: Contact phone/fax, email address: After hours phone/fax: Address for correspondence: Declaration In signing I certify that I am aware of my responsibilities as set out in the Animal Research Act 1985 and Regulation 2010 and the Australian code of practice for the care and use of animals for scientific purposes. Full name of signatory: Signature: Date: Name: Role: Student Investigator Investigator Professional Staff Contact phone/fax, email address: After hours phone/fax: Address for correspondence: Declaration In signing I certify that I am aware of my responsibilities as set out in the Animal Research Act 1985 and Regulation 2010 and the Australian code of practice for the care and use of animals for scientific purposes. Full name of signatory: Signature: Date: Note: Copy and paste additional entries, as necessary. 3.3 Declaration by Head of School / Institute Director In signing I certify that the project is appropriate to the general facilities available and that I am prepared to have the project carried out in my School/Institute in accordance with the Animal Research Act 1985 and Regulation 2010 and the Australian code of practice for the care and use of animals for scientific purposes. Full name of signatory: Signature: Date: 116105270 Page 3 4. Is this a new project? ......................................................................... Yes No 5. A replication of a previously conducted study? ................................ Yes No 6. Complete the table below. Refer to the Summary of Codes as required. Purpose 7. Procedure Species category Total number of animals A project which has (previously or simultaneously) been submitted to this or another ethics committee? .............................................................................................................. Yes No Yes No If Yes, provide reasons for re-submission or simultaneous submission and the name of the ACEC(s). 8. Has an application for this or a similar project been refused by this or another ACEC? .... If Yes, provide details: 9. A significantly revised current protocol? ......................................................................... Yes No If Yes, quote the approval number, species and number of animals used to date? 10. Have any of the people participating in this project had any animal research authority or animal supplier’s licence cancelled? ......................................................................................................... Yes No If Yes, provide details including the name of the person, the date on which the authority or licence was cancelled, who cancelled the authority or licence and the reason for the cancellation. 11. Does this project involve collaboration with an external organisation or agency (i.e. institution, company, individuals etc)? ............................................................................................................ Yes No If Yes, please provide the name of the collaborator, details of the collaboration. 12. Source and grant identifier number of project funds. Please include one copy of the grant application when you submit this application to the ACEC. 116105270 Page 4 13. Type of Activity and Field of Research Classification Complete the tables below. Refer to Fields of Research Classification for the 6 digit classification number. Type of Activity Percentage (%) Field of Research Percentage (%) Basic Strategic Applied Experimental 14. Does this proposal involve the use of native, imported and /or protected species? ...................................................................................................... If Yes, please tick the relevant box: Native Imported Yes No Protected. 15. Name the issuing body and permit numbers for any special permits obtained for this project: Are copies attached?..................................................................................... ............. Yes No Yes No 16. Animal Supplier Details: Name of licensed animal supplier Address 17. Are non-exempt animals (eg. Farm cattle, sheep) to be used?………………………. If Yes, indicate the source / supplier of the animals: 18. Project duration (indicate the propose date (dd/mm/yy) Proposed commencement date Proposed date of completion Animal arrival date 116105270 Page 5 Animal disposal date B. OVERVIEW OF PROJECT 1. Describe the project aims in lay (non-scientific language) terms 2. Rationale Provide a justification for the use of animals. This should be based on an assessment of the scientific /educational value and the potential impact on the welfare of the animal. 3. Outcomes/ Benefits of the project in lay terms (non-scientific language) Specify what you hope to achieve. 4. Summarise the research plan Include the project’s empirical and theoretical background, time line for animals, procedures to be used, data gathering/fieldwork and data analysis. 116105270 Page 6 C. ETHICAL CONSIDERATIONS Russell and Burch’s principles of Replacement, Reduction and Refinement (most commonly referred to as the 3R’s) provide a systematic framework to achieve the goal of humane experimental techniques. Replacement is defined as the substitution for conscious living higher animals of insentient material. The goal of reduction is to reduce the numbers of animals used to obtain information of a given amount and precision. Refinement is any decrease in the incidence or severity of 'inhumane' procedures applied to those animals that still have to be used. Your proposal must demonstrate how the 3R’s are being addressed. Replacement 1. Replacement: Provide an explanation of why animals are needed for the project, including a list of potential alternatives to animal use, whether any alternatives would be used and if not why alternatives are unsuitable. Reduction 2. Complete the table below regarding the proposed animal usage Species# # Strain Number/year Stocking rate As per the Summary of Codes description. 3. Is the number of animals to be used (and where appropriate by treatment groups) the minimum that is appropriate to answer the question being posed? 4. Justify why this number is necessary, including whether the proposal has been the subject of previous research and if so why repetition is necessary? 5. Will animal tissue be shared with other investigators? 116105270 Page 7 6. Sequence of events Your proposal must give a step by step description of what happens to each animal from acquisition to disposal. Please attach a flow chart or sequence of events table. Appendices should show project title and question number. Impact 7. Please identify all experimental and other procedures that may impact on an animal’s well being. Refinement 8. Provide details of the refinement of procedures that reduce the adverse impact on animals. Fate of Animals 9. What is the fate of animals at completion of project? If animals are to be euthanased, how will this be done, where will it be carried out, who will do it (including experience in the technique to be used)? 10. Animal Disposal Details – Complete the table below: Name of abattoir/disposal company Address 116105270 Page 8 D. ANIMAL MONITORING, HOUSING AND MANAGEMENT Animal Monitoring: How any impact will be monitored, assessed and managed and procedures to identify and quickly respond to unforseen complications. 1. How will animals be identified? 2. Who will monitor the animals? (Include names, contact details, qualifications, and experience with the species being used). 3. On a day-to-day basis: 4. At night, on weekends and holidays: 5. For those procedures and treatments that have been identified as having an impact on the animal’s wellbeing, detail the method and frequency of monitoring animals during and after procedures / educational activities are carried out. You may attach a monitoring sheet that reflects your attention to these signs. 6. Method and frequency of monitoring animals during housing. 7. Please identify all locations, where the animals will be housed and where procedures on animals will be performed. Full street address should be supplied where applicable. If field based, name the properties or closest town. 8. What type of housing will be provided? 9. What is the maximum and minimum number of animals per cage/pen? 10. What and how often will the animal be fed? 11. What is the diet? 12. What is the feeding method and frequency? 13. How is water provided? 14. What environment enrichment will be provided? 15. Other husbandry procedures (eg. foot trimming). 116105270 Page 9 16. Provide details of what will be done if a problem is identified, including criteria for intervention, treatment or withdrawal of the animals from the proposal. 17. Who is responsible for the management of emergencies and how will you ensure that the nominees can be contacted? 18. What is the maximum time an individual animal is held? 19. Does the project involve the use of animals that have been the subject of a previous experiment? If YES, what was previously done to these animals? (Include project number and experiment description). E. OTHER CONSIDERATIONS Work, Health and Safety - Risk Assessment and Management 1. Identify any infectious diseases (zoonoses) that may be transferred to staff or students. 2. Have steps been taken to: Control and/or eliminate the risks identified above? ............................................. Yes Special Ethical Considerations 3. Please identify any features of the project, which raise special ethical considerations. 116105270 Page 10 No NA F. ADDITIONAL INFORMATION FOR FIELD BASED PROGRAMS Does this application involve field based programs? ........................................................ Yes No If Yes, continue with completing Section F. 1. What species of animal will be used? 2. Are there specific target species? ..................................................................................... . Yes No 3. If Yes to Q2, what are the target species? 4. Why is it necessary to capture animals? 5. What alternatives to capturing animals could be used? 6. What precautions will be taken if lactating animals or animals with pouch young are captured? 7. Have the guidelines found at Wildlife Research published by the Animal Research Review Panel (NSW DPI) been consulted in order to minimise the impact on animals? ................................................ Yes No 8. If not, why not? 9. How many traps will be set and over what period of time? 10. State the maximum number of traps per investigator that will be set. 11. What traps will be used? Pitfall traps should only be used after consulting, Use of pitfall traps by ARRP (NSW DPI). 12. If using mist nets, please detail experience and training in use. 13. How often and at what times will traps be checked and/or cleared? 14. Describe any other methods to be used for capture. 15. What precautions will be taken in the event of inclement weather? 16. Will samples be taken, eg. milk, hair, scales? ................................................................. Yes No Yes No 17. If Yes to Q16, what samples will be taken? 18. How will samples be taken? 19. How will animals be restrained or handled? 20. Is transportation necessary? ............................................................................................ 21. If Yes to Q20, how will animals be transported, over what period of time and what precautions will be taken against cold/heat stress? 22. Will animals be marked for individual identification? ........................................................ Yes No 23. If Yes to Q22, how will they be marked? 24. What, if any, adverse effects will there be on the animals in relation to all the procedures you will carry out? 25. If invasive methods are to be used, how will pain /distress be minimised? 116105270 Page 11 26. Will any radio tracking collars or other radio tracking equipment be used? ..................... Yes No 27. If Yes to Q26, what equipment will be used on the animal, how will it be attached, what is the weight of the equipment and the impact on the animal? 28. How will the equipment be retrieved? 29. Anything you care to add? 116105270 Page 12 Attachment 1: Technical Competence For assistance in completing this attachment, refer Technical Competence - Guidelines Section 1: Document version and project title Project Title ACEC Reference Chief Investigator Investigator Investigator Investigator Section 2: Procedures (Including but not limited to animal handling, surgery, anaesthesia and euthanasia) Procedure Animal Handling* *Animal handling is species-specific and is a required field for all investigators. Section 3: Experience and competency with listed procedures Name Procedure to be Level of experience or proposed training with stated performed by procedure and species this investigator Does the CI certify that this investigator can competently perform the procedure? Yes/No Animal Handling* *Mandatory field for all investigators 116105270 Page 13 Date







