Solution
advertisement
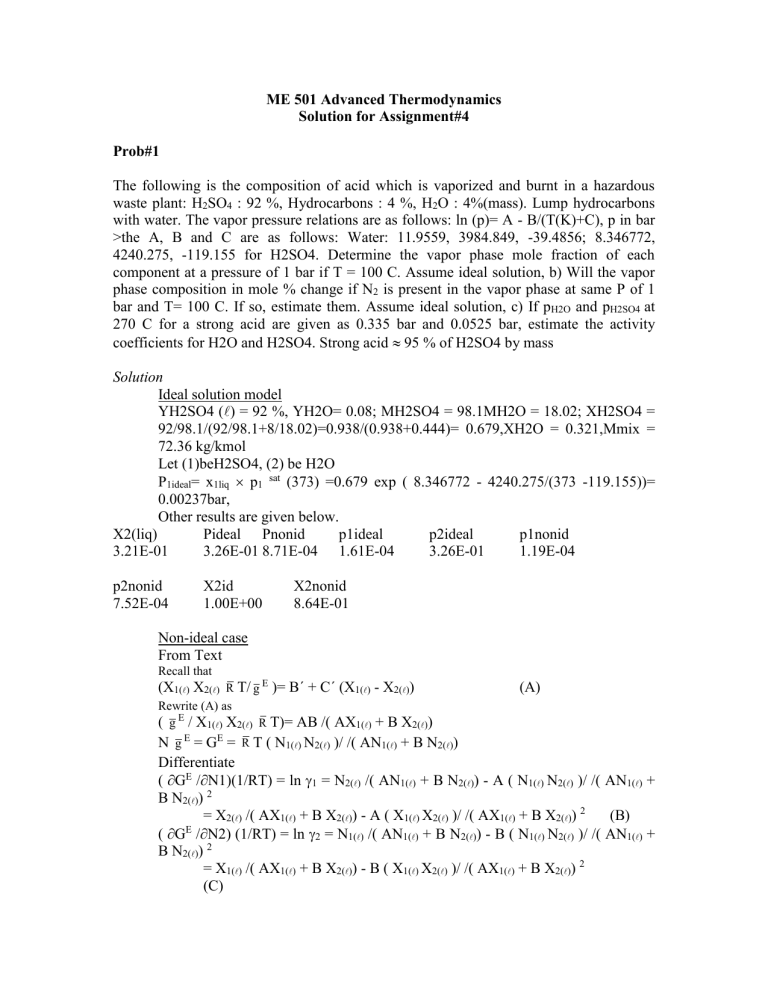
ME 501 Advanced Thermodynamics Solution for Assignment#4 Prob#1 The following is the composition of acid which is vaporized and burnt in a hazardous waste plant: H2SO4 : 92 %, Hydrocarbons : 4 %, H2O : 4%(mass). Lump hydrocarbons with water. The vapor pressure relations are as follows: ln (p)= A - B/(T(K)+C), p in bar >the A, B and C are as follows: Water: 11.9559, 3984.849, -39.4856; 8.346772, 4240.275, -119.155 for H2SO4. Determine the vapor phase mole fraction of each component at a pressure of 1 bar if T = 100 C. Assume ideal solution, b) Will the vapor phase composition in mole % change if N2 is present in the vapor phase at same P of 1 bar and T= 100 C. If so, estimate them. Assume ideal solution, c) If pH2O and pH2SO4 at 270 C for a strong acid are given as 0.335 bar and 0.0525 bar, estimate the activity coefficients for H2O and H2SO4. Strong acid 95 % of H2SO4 by mass Solution Ideal solution model YH2SO4 () = 92 %, YH2O= 0.08; MH2SO4 = 98.1MH2O = 18.02; XH2SO4 = 92/98.1/(92/98.1+8/18.02)=0.938/(0.938+0.444)= 0.679,XH2O = 0.321,Mmix = 72.36 kg/kmol Let (1)beH2SO4, (2) be H2O P1ideal= x1liq p1 sat (373) =0.679 exp ( 8.346772 - 4240.275/(373 -119.155))= 0.00237bar, Other results are given below. X2(liq) Pideal Pnonid p1ideal p2ideal p1nonid 3.21E-01 3.26E-01 8.71E-04 1.61E-04 3.26E-01 1.19E-04 p2nonid 7.52E-04 X2id 1.00E+00 X2nonid 8.64E-01 Non-ideal case From Text Recall that (X1() X2() R T/ g E )= B´ + C´ (X1() - X2()) Rewrite (A) as ( g E / X1() X2() R T)= AB /( (A) AX1() + B X2()) N g = G = R T ( N1() N2() )/ /( AN1() + B N2()) Differentiate ( GE /N1)(1/RT) = ln 1 = N2() /( AN1() + B N2()) - A ( N1() N2() )/ /( AN1() + B N2()) 2 = X2() /( AX1() + B X2()) - A ( X1() X2() )/ /( AX1() + B X2()) 2 (B) E ( G /N2) (1/RT) = ln 2 = N1() /( AN1() + B N2()) - B ( N1() N2() )/ /( AN1() + B N2()) 2 = X1() /( AX1() + B X2()) - B ( X1() X2() )/ /( AX1() + B X2()) 2 (C) E E Solve for A and B from Eqs. (B) and (C) ln 1 = A X2()2/((A/B) X1() + X2())2, ln 2 = B X1()2/((X1() + (B/A) X2())2 At 95 % H2SO4, XH2SO4(liq) = (D) 0.777288413, XH2O(liq) = 0.222711587 P1 non-deal= 1 x1liq p1 sat (503)= 1 p1ideal (543, Y=xH2SO4= 0.78) where p1ideal = 0.777 exp ( 8.346772 - 4240.275/(543 -119.155)) P1ideal = 0.148683064, p2ideal= 12.70896563 bar 0.0525/0.149 = 1 ( 544,0.,78) gamma1 = 0.353100069, gamma2= 0.026359344. Using these gammas, A = ln 1() (1+ (X2() ln 2())/(X1() ln 1()))2, (1) B= ln 2() (1+ (X1() ln 1())/(X2() ln 2()))2. (2) One can obtain A and B as -14.53288172, -4.167124513 Using Eqs. (51a,b) in ln 1 = A(T) X2()2/((A(T)/B(T)) X1() + X2())2, ln 2 = B(T) X1()2/((X1() + (B(T)/A(T)) X2())2, Treating A as constant and with X1liq = 0.679, X2liq = 0.321, 1 and 2 are obtained. p1nonid = 1 p1ideal ( 100 C, XH2SO4 =0.679) = 1.19E-04 Results below: X2(liq) Pideal Pnonid p1ideal p2ideal 1 3.21E-01 3.26E-01 1.30E-02 1.61E-04 3.95E-02 8.13E-01 2 p1nonid p2nonid X2id X2nonid 3.95E-02 1.31E-04 1.29E-02 1.00E+00 9.90E-01 Prob#2 Suppose we have 7 g mole of methanol (1) ( liquid + vapor) and 3 g mole of water (2) (liquid + vapor) in a piston cylinder assembly at 60 C, 433 kPa. At T= 60 C, p 1sat = 625 mm of Hg, p2sat = 144 mm of Hg. Determine a) x1, x2, Y1, Y2 b) vapor fraction or quality ¯,w c) moles of vapor of species 1 and 2 Solution a) P = p1 + p2 = x1 p1 sat + x2 p2 sat = x1 p1 sat + (1-x1 ) p2 sat Solving for x1 = 0.6, x2 = 0.4 Y1 = p1 /P =x1 p1 sat /P = 0.83, Y2 = 0.13 b) ¯,w= Ng /N ? N1 = N1 + N1g Dividing by N Z1 = N /N + N1g/N = (N /N ) (N /N) + (N1g /Ng) (Ng/N) 0.7 = x1 (1- ¯,w) + Y1 ¯,w = 0.4 (1-¯,w) + 0.83 ¯,w, Solving for ¯,w = 0.698 c) Ng = 0.698 10 = 6.98 g moles, liquid moles will be 10 -6.98 = 3.02 N = 0.6 3.02 =1.81, Similarly N = 1.21, N1g = 0.83 6.98 = 5.79, N2g = 1.19. Prob#3 Determine the maximum temperatures to which liquid water can be superheated and vapor can be subcooled at 133 bar. Solution a) P = RT/(v-b) - a /(Tnv2) At points M and G, ∂P/∂v = - RT/(v-b)2 + 2a/(Tn v3) = 0 RT(n+1) v3 = 2a (v-b)2 Solve for v at given T. Obtain the two solutions vM, vG. Knowing vM and vG we can obtain the spinodal pressures PM and PG from Eq.(A). We can also get the spinodal curve P vs. v by eliminating T between equations (B) and (A). Thus for the case n = 0 (VW equation of state), P = a(v-2b)/v3 (Note :If v < 2b, then P < 0 from Eq. (B). The negative pressure implies tensile stress on the fluid. Recall that b = vc /3 for VW; thus for v > 2vc/3, P > 0 ; otherwise tensile stress may be existing). b) In reduced form for VW TR = 54/64 ( vR' - 0.125)2 / vR' 3 and PR = ((27/64)/vR' 2 ) (1 - (1/(4 vR'))) See Fig. for the spinodal curve based on VW equation of state. c) ln PR sat - 1/TR); TR = 7/(7- ln PR sat) = 7/(7- ln (133/220.9)) = 0.932 T = TR Tc = 0.932647.3 = 603.6 K P = 133 bar, vA = 0.069, vB = 0.16 m3 /kmol, TA = 600 K (?), TB = 545 K, Tsat = 604 K according VW equation of state. Tsuperheat =, Tsubcool = -55 K (A) (B)