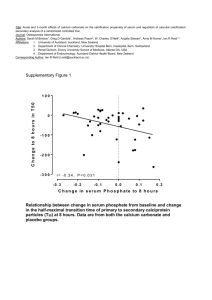
17_Cooper\Beta cell Chapter INDEXED
advertisement

‘gel-free’-based separation methods, 7 ‘organelle-specific’ proteomic analyses, 25 aggregation, 16, 23, 26 amylin, 5, 8, 9, 12, 13, 14, 16, 17, 18, 26, 27, 28, 38, 39, 42, 43, 44, 45 amylin aggregation, 17 amylin misfolding, 27 amylin-evoked apoptosis, 16 amylin-generated aggregates, 28 amylin-mediated, 17, 18 amyloid, 16, 17, 18, 28, 29, 38, 39, 41, 42, 43 amyloid fibrils, 17 anti-diabetic compounds, 28 apoptosis, 16 ATF2/p38 MAPK, 17 autoantigens, 10, 12 autoimmune destruction of islet β-cells, 10 autoreactive T-cells, 10 B-cell secretory granules, 10, 14, 25 bioinformatic, 21, 22 Ca2+, 11, 12, 14, 15, 25, 40 Ca2+ efflux, 11 calcium storage, 12 calreticulin, 20, 24, 25, 40 caspase-3, 17 caspase-8, 17 chaperone, 23, 24, 26, 27, 41 chaperones, 23, 27, 28, 41 composition of the β-cell granule, 9 co-purification, 26 C-peptide, 14 cytotoxic oligomers, 9 cytotoxic processes, 28 cytotoxic protein aggregates, 9 decreased β-cell mass, 17 defective insulin secretion, 11, 12 degeneration of the islets of Langerhans, 8 diabetes, 4, 5, 8, 9, 12, 13, 16, 17, 27, 29, 37, 38, 39, 42, 43, 44 disease mechanisms, 5, 7, 8, 13, 27 disease processes, 12 disorders of hormone action, 8 dysregulated amylin folding, 28 dysregulation of pancreatic hormones, 8 electron microscopy, 14 ER, 3, 11, 17, 18, 20, 21, 23, 24, 25, 27 exocytosis, 11, 14, 15, 26, 41 experimental therapeutics, 7 Fas/FasL/FADD, 16, 17, 18 fibrillogenic, 17 fibrils, 16 fuel metabolism, 18 glucagon, 8, 45 glutamate decarboxylase (GAD), 10 glycoisoforms, 7 granule proteins, 5, 10 granule proteome, 5 halo, 13 heat shock protein, 22 heat-shock protein 65 (HSP65), 10 high-resolution methodologies, 5 HSP, 27 human amylin transgenic mice, 17 hyperglycaemia, 17, 28 immunoaffinity purification, 24 immunopurified, 21 in-gel tryptic digestion, 20 INS-1E, 20, 21, 46 insoluble fibrils, 28 insulin, 5, 8, 9, 10, 11, 12, 13, 14, 15, 18, 20, 21, 24, 25, 26, 28, 38, 39, 40, 41, 43, 45, 46 insulin resistance, 18 insulin resistant, 8 insulin therapy, 9 intracellular Ca2+, 11 islet amyloid, 8, 16, 28, 37, 38, 39, 43 islet amyloidosis, 28, 38 islet hormone production and release, 8 islet hormones, 18 islet structure and function, 29 islet β-cell degeneration, 16, 17 islet β-cell dysfunction, 26 islet-cell antigen 69 (ICA69), 10 islet-specific proteins, 18 isobaric tags for relative and absolute quantitation (iTRAQ), 8 JNK1/cJun, 17 LC-MS2, 21, 22 liquid chromatography (LC), 7 lysosome, 20, 23 lysosomes, 21, 46 misfolding, 9, 12, 16, 18, 23, 26, 27, 28 mitochondria, 20, 21, 22, 25, 26, 41, 46 mitochondrial ATP synthase, 26 molecular mechanisms, 12 molecular pathways, 9 multi-dimensional mass spectrometry, 7 multi-dimensional protein identification technology (MuDPIT), 8 NAADP, 11, 12 nicotinic acid adenine dinucleotide phosphate (NAADP), 11 novel islet T-cell antigens, 10 oligomer formation, 16 oligomeric, 7 oligomers, 16, 26, 28 p53/p21WAF1/CIP1, 17 pancreas, 8, 9, 15, 39, 43, 45 pancreatic islet β-cell, 3, 5, 18, 26 pancreatic islets, 8, 15, 27 pancreatic β-cells, 27 pathogenesis of T2DM, 11, 16 Pdx1, 19 pharmacotherapies, 5 physiological processes, 5 post-translational modifications, 6 proinsulin, 14, 15, 40, 41 protein folding, 9 protein identification analysis, 20, 21 proteome, 6, 8, 18, 20, 21, 29, 39, 40 proteomic, 5 proteomic analysis, 3, 5, 6, 9, 12, 15, 19, 27, 28, 29, 43 proteomic methods, 7 proteomics, 6, 8, 12, 15, 16, 18, 19, 23, 41 protofibrils, 16, 28 PTM, 3, 7 PTMs, 6, 7 purification steps, 20 Rab GTPases, 11 recombinant human insulin, 15 regulated insulin secretion, 12 regulated secretion, 5, 8, 12, 28 regulation of fuel metabolism, 8 regulation of insulin and amylin secretion, 12 regulation of islet hormone secretion, 28 regulation of metabolism, 5, 8 role of insulin, 19 ryanodine receptor (RyR) I, 11 RyR, 3, 11 SDS-PAGE, 20 secretory granule, 3, 5, 6, 8, 9, 10, 11, 12, 13, 15, 18, 19, 20, 23, 24, 26, 28 secretory vesicle, 12 semi-quantitative comparisons, 8 subcellular, 11, 13, 23, 27 subproteomes, 6 T1DM, 3, 9, 10, 28 T2DM, 4, 9, 10, 12, 16, 17, 18, 26, 28, 29 T-cell, 10 therapeutic interventions for diabetes, 13 T-lymphocytes, 10, 25 transgenic models of amylin-mediated diabetes, 17 two-dimensional gel electrophoresis (2DGE), 7 type-1 diabetes (T1DM), 9 type-2 diabetes (T2DM), 9 ultracentrifugation, 20, 21 VAMP, 13 VAMP2, 11 western blotting, 20 zinc-containing crystals, 13 Zn2+, 24 β-cell, 6, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 23, 24, 25, 26, 27, 28, 29, 38, 39 β-cell death, 28 β-cell degeneration, 9, 17, 23, 29 β-cell dysfunction, 26, 29 Β-cell granule proteins, 5 β-cell granule-specific T-cell lines, 10 β-cell secretory granules, 10, 11, 13, 14, 19, 20, 21, 25, 27, 28 β-conformers, 16 β-sheet, 16, 26 Title: Proteomic analysis of the pancreatic islet β-cell secretory granule: current understanding and future opportunities Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 2 Au: Garth J S Cooper School of Biological Sciences Faculty of Science University of Auckland NEW ZEALAND Email:g.cooper@auckland.ac.nz and Department of Pharmacology, Division of Medical Sciences University of Oxford Mansfield Road Oxford OX1 3QT UNITED KINGDOM Email: garth.cooper@pharm.ox.ac.uk Nonstandard abbreviations: 2DGE, two-dimensional gel electrophoresis; AFM, atomic force microscopy; ER, endoplasmic reticulum; FasL, Fas ligand; FADD, Fas-associated death domain protein; GAD, glutamate decarboxylase; HSP, heat-shock protein; ICA, islet-cell antigen; iTRAQ, isobaric tags for relative and absolute quantitation; MALDI-TOF, matrix-assisted laserdesorption-ionization time-of-flight; MS, mass spectrometry; MuDPIT, multi-dimensional protein identification technology; NAADP, nicotinic acid adenine dinucleotide phosphate; PC1, proprotein convertase 1; PTM, post-translational modification; RyR, ryanodine receptor; T1DM, type-1 diabetes mellitus; T2DM, type-2 diabetes mellitus; VAMP, vesicle-associated membrane protein Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 3 Chapter 17 Proteomic analysis of the pancreatic islet β-cell secretory granule: current understanding and future possibilities Garth J S Cooper “To every complex question there’s a simple answer, and it’s always wrong” H. L. Mencken (attrib.) Abstract ― The pancreatic islet β-cell granule has been the subject of intense study for decades, in part because it serves as the vehicle for the regulated secretion of insulin and amylin, through which it exerts regulation of metabolism. Β-cell granule proteins have been closely linked to disease mechanisms in both major types of diabetes, and recent findings from genome-wide association studies have reinforced the importance of these linkages for understanding disease mechanisms. Granule proteins have also proven to be of major interest in pharmaceutics, since two of them, insulin and amylin have each served as the basis for the development of antidiabetic pharmacotherapies. In spite of all the attention this enigmatic granule has received to date, many fundamental questions about its molecular structure and function remain unanswered. In the past few years, high-resolution methodologies have begun to unravel the granule proteome in ever-increasing detail. Emerging data complements the results from the other approaches that have been applied to understand the granule. This chapter will explore the current state of knowledge in the field, and the implications of emerging proteomic data for the study of physiological processes and disease mechanisms in diabetes. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 4 1. Introduction: proteomics and the β-cell secretory granule 1.1 Proteomes and proteomics: definitions The proteome may be defined as the complete set of proteins expressed by a genome, cell, tissue or organism. Proteomes typically vary according to developmental stage and in response to environmental or genetic influences. Subproteomes may also be defined. They may, for example, comprise all the metalbinding proteins, the phospho-proteins, the glycosylated proteins, or the membrane proteins expressed by an organism, organ, tissue, cell or organelle, to mention a few of the myriad possibilities. There are clearly many different ways in which such subproteomes can be delineated. Proteomics is the study of proteomes. Proteomic analysis frequently begins with the study of whole organs or tissues, and then, according to the findings and emerging focus, proceeds to the examination of sub-cellular fractions or organelles, for example the ‘mitochondrial proteome’, with increasing degrees of resolution [1]. Proteomics is frequently employed in the first instance in its so-called ‘hypothesis-generating’ or ‘hypothesis-free’ mode, where it can be extremely effective in generating hypotheses, for example by comparisons between related states [2]. Thereafter, it can be switched into its ‘hypothesis-driven’ mode, where hypotheses generated from analysis of the first phase of investigation can be explored in ever-increasing detail in a series of follow-up experimental designs. Amongst its many beneficial properties, proteomics is particular adept at identifying and characterising post-translational modifications (PTMs) in proteins [3, 4]. This ability to detect Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 5 and quantify the contributions made to the modification of protein structure by the many possible post-translational modifications, is now pointing the way towards levels of regulation in biological systems that are far more complex than has previously been envisaged [5, 6]. The multiple, regulated glycoisoforms of the protein adiponectin and the variable oligomeric structures that they generate, provide a useful example of the complexity that is increasingly being unveiled by the systematic application of proteomic PTM analysis [2, 7, 8]. In future, proteomic PTM investigation is expected to contribute substantively to our understanding of disease aetiopathogenesis, and to deliver many new targets for research into disease mechanisms and the generation of experimental therapeutics [3]. 1.2 Proteomic methods: a very brief overview Previously, proteomic methods frequently employed two-dimensional gel electrophoresis (2DGE) to perform the required separation of complex mixtures of proteins, followed by multi-dimensional mass spectrometry and informatics for protein identification. Although 2DGE-based methods can yield important information [9, 10], for example through the detection and characterisation of groups of closely-related proteins that differ in the quantity or quality of their similar PTMs [4, 11], they are also subject to the many shortcomings imposed by the physical properties of the many classes of proteins that cannot be resolved adequately using gels (for example, those with very high or low molecular weights, membrane proteins, those with high or low pI values, fibrous, very large or crosslinked proteins such as those occurring in the ECM, and those of low abundance). These limitations mean that most proteins present in most proteomes are inaccessible to analysis by 2DGE-based methods. Therefore there has been an increasing shift towards liquid chromatography (LC)- or ‘gel-free’-based separation methods applied to proteolytic digests of whole proteomes with Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 6 subsequent fractionation and labelling, for example those based on multi-dimensional protein identification technology (MuDPIT). These approaches have the additional advantage that, with the introduction of approaches such as isobaric tags for relative and absolute quantitation (iTRAQ), semi-quantitative comparisons between multiple related proteomes have become feasible (for examples, see [12, 13]). 1.3 The islet β-cell and its secretory granule: targets for proteomics What is the possible relevance of proteomics to the insulin secretory granule? The pancreatic islets play a key role in the regulation of metabolism through their regulated secretion of the peptide hormones insulin, amylin, and glucagon. They are of fundamental interest in the study of a broad range of disease mechanisms, including those characterised by dysregulation of pancreatic hormones as well as by disorders of hormone action, such as occur in insulin resistant states [14]. Proteomic investigation of the pancreas has been motivated by several objectives. One of these is to improve our understanding of the mechanisms of islet hormone production and release, and their linkages to the regulation of fuel metabolism [14]. Another is to identify proteins and, through them, processes that might provide better understanding of diseases that directly impact on the pancreatic tissues, chief amongst which are diabetes mellitus, pancreatitis and pancreatic cancer [15-22]. At one level, investigation of the pancreatic proteome has arguably been underway for most of the last hundred years, driven in large part by the need to understand and reverse the processes that lead to or cause diabetes. Islet amyloid or ‘hyaline’ (Figure 1) was the original observation that linked degeneration of the islets of Langerhans to the causation of the form of Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 7 the disease now known as type-2 diabetes (T2DM) [23, 24]. Fundamentally important results from early protein chemical studies of the pancreas led to the isolation and characterisation of insulin, and the development of insulin therapy, initially for type-1 diabetes (T1DM), and later for T2DM [25]. Decades later, granule proteins were identified as likely targets of immune mechanisms related to the aetiology and pathogenesis of T1DM [26]. (Figure 1 near here) 1.4 Granule-associated pathogenic processes and the origins of diabetes Increasing evidence has implicated misfolding of the β-cell hormone amylin [27-29] to generate cytotoxic oligomers [30, 31], as potentially responsible for β-cell degeneration in T2DM [32]. These phenomena provide a clear rationale for elucidation of the composition of the β-cell granule at high resolution, with the aim of indentifying intrinsic molecular pathways that might mediate as-yet unknown granule functions [20, 22] – for example those relating to the control of protein folding within the granule, and possible defects that might contribute to the formation of cytotoxic protein aggregates [20, 33]. Evidence underpinning facets of this emerging pathogenetic mechanism is developed in the following section, to provide an example of but one of the important unsolved mysteries of the β-cell secretory granule, which may prove amenable to proteomic study. There are at least two other well-recognized pathobiological questions of fundamental importance relating to the granule, where proteomic analysis could also have a part to play. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 8 1.5 Granule proteins as putative autoantigens in T1DM The first of these questions relates to the nature of the component proteins that act as autoantigens in the autoimmune destruction of islet β-cells, for example in T1DM. Numbers of proteins have been identified as candidate autoantigens with potential relevance to the mechanisms of autoimmune β-cell destruction, either through studies in animal models such as the non-obese diabetic (NOD) mouse, or in human patients [34-37]. Most are components of β-cell secretory granules, although they may also exist in other organelles, and frequently in other cell types as well. Several of these candidate autoantigens are recognized by T-lymphocytes, including insulin, glutamate decarboxylase (GAD) 65 and GAD 67, heat-shock protein 65 (HSP65), and islet-cell antigen 69 (ICA69) [38]. Nevertheless, there remains uncertainty concerning the nature of another group of autoantigens associated with the secretory granule [39]. Indeed, there is evidence for recognition of novel islet T-cell antigens by β-cell granule-specific T-cell lines from new-onset T1DM patients, where a fraction of islet β-cells appear to be targeted predominantly by autoreactive T-cells [39]. These considerations point to a need for improved understanding of the protein components of the secretory granule for the following reasons: (i), to identify new potential autoantigens, and (ii), to better elucidate the mechanisms that evoke T-cell activation and T-cellmediated autoimmune β-cell destruction in T1DM. 1.6 Granule proteins and hormone secretion The second question relates to defective insulin secretion in T2DM. B-cell secretory granules have been studied for decades, with the major aim of elucidating the mechanisms of insulin processing and secretion [40-42]. Recent results from Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 9 genome-wide association studies (GWAs) [43] have reinforced linkages between defective insulin secretion, β-cell granule proteins, and the pathogenesis of T2DM [44, 45]. Many studies of the role of β-cell granules in insulin secretion have focussed on the mechanisms by which insulin is processed and stored [41, 42], and the roles of ion channels [4648] and other proteins in the regulation of their exocytosis [49-58]. Some of the proteins that mediate exocytosis, for example the small Rab GTPases and VAMP2, are intrinsic to substructures within the β-cell secretory granule [59, 60], whereas others reside in other parts of the cell and may therefore not co-purify with granules. In recent years, increasing evidence has pointed to the secretory granule itself as playing a leading role in the triggering of its own secretion. In particular, ryanodine receptor (RyR) Imediated Ca2+-induced Ca2+ release from the β-cell secretory granule, possibly potentiated by nicotinic acid adenine dinucleotide phosphate (NAADP), is increasingly seen to play an essential role in the activation of insulin secretion [61, 62]. Receptors for NAADP, a novel intracellular Ca2+-mobilizing agent [63, 64], may represent an alternative pathway for Ca2+ efflux from β-cell secretory granules [65]. Islets and MIN6 β-cells express two RyR isoforms, RyR I and RyR II, which display distinct subcellular localizations. Whereas type-I RyRs were present in approximately equal density in a mixed vesicle/mitochondrial fraction and in microsomes, RyR II was considerably more abundant on ER membranes [62]. Functional NAADP-sensitive Ca2+stores are also present in human β-cells [66]. Dantrolene, a selective inhibitor of RyR I, increased steady-state free [Ca2+] in β-cell secretory granules but not in the ER, consistent with the presence on granules of a further activator or channel capable of amplifying the effects of RyRs on Ca2+ release. Receptors for Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 10 NAADP may thus serve this role, and insulin secretory vesicles, but not the ER, may comprise an NAADP-responsive Ca2+ store [62]. Regulated calcium storage in the insulin secretory granule has thus been implicated in the mechanisms of regulated insulin secretion. This emerging picture implicates Ca2+ regulation within the insulin secretory vesicle itself as pivotal to the regulated secretion of insulin and amylin, and points to areas where proteomic analysis might be able to contribute to the elucidation of molecular mechanisms. Questions that arise include those of the nature of the proteins that might mediate aspects of this emerging process, the nature and roles of putative Ca2+-binding proteins in the insulin secretory granule, and ultimately the nature of the molecular defects in T2DM that generate defective insulin secretion. In order to understand such processes at the molecular level, it would help to know which proteins and pathways are actually present in the granule, so as to understand which may possibly be implicated in disease processes that might occur therein. 1.7 Potential future contributions by proteomics Phenomena, such as those which potentially link aspects of insulin secretory granule function to the misfolding of amylin, the regulation of insulin and amylin secretion, the generation of β-cell autoantigens, and through these processes to the pathogenesis of the major types of diabetes, provide a clear motivation and focus for the systematic, ongoing investigation of this organelle. Proteomic analysis is but one of a number of hypothesis-generating methodologies that are now being brought to bear on questions concerning the origins and mechanisms of diabetes, and the roles of granule-associated pathways in these processes. One of its advantages in this Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 11 case is that it can be selectively targetted at the granule itself, as explained below. Other hypothesis-generating methods include GWAs, whole-genome transcriptomics, and metabolomics, which together contribute different aspects of the information available in the broader field of systems biology, are broader but less focussed in their scope. One of the challenges that will need to be met in the next phase of the application of systems biology to the insulin secretory granule, is the integration and interpretation of the large data sets currently being generated by these complementary but distinct methodologies, with the objective of generating testable hypotheses for the targetted dissection of disease mechanisms, and the use of these in the generation of new, integrated hypotheses whose final goal must be the generation of new and improved therapeutic interventions for diabetes. 2. β-cell secretory granules : structural regions and functional specialization The β-cell granule performs a specialized subcellular function in the storage and secretion of insulin and amylin. It is a complex intracellular organelle containing many proteins with different catalytic activities and messenger functions [24, 67, 68] along with other components including adenine nucleotides, inorganic phosphate and bivalent metal ions [69]. The granule itself comprises several distinct structural regions, including the dense core with its component insulin- and zinc-containing crystals, the halo, and the enveloping, VAMPcontaining outer membrane [60, 68, 70-72]. There is evidence for differential distribution of component proteins between these different regions, which subserve distinct functions. These different structures could possibly be targetted individually in future proteomic studies, with consequent increases in resolution and improved understanding of protein distribution and function within the granule. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 12 B-cell secretory granules can be visualized by electron microscopy as spheroidal structures of about 200-300 nm in diameter (Figure 2), and comprise a crystalline core of zinc/insulin-containing crystals surrounded by a mantle of less dense material, enwrapped by a phospholipid bilayer membrane [68, 69]. The granule is, however, far more than just a cellular repository for processed insulin. For example, its membrane contains a series of proteins involved in the integration of its trafficking and docking to the cell membrane and (as discussed above), it contributes actively to its own secretion through regulation of cell-Ca2+ metabolism [64]. In the early 1980s, Hutton reported that β-cell granules may contain more than 150 distinguishable proteins in addition to their major constituents, which were considered to be insulin and its connecting-peptide (C-peptide) [73], and that a number of these are secreted in addition to insulin [74]. Additional granule components were noted to include proteinases implicated in proinsulin-to-insulin conversion, intermediates in that conversion process, minor co-secreted peptides, membrane proteins that mediate granule movement and exocytosis, and ion-translocating proteins involved in the regulation of the within-granule environment. More recently, amylin (designated also as IAPP), was found to be a second major hormone packaged in the β-cell secretory granules , which is also mainly β-cell-specific [27, 75]. Interestingly, amylin may be predominantly present in the granule haloes, whereas processed insulin resides mainly in the dense cores. (Figure 2 near here) Typical β-cells contain about 104 insulin secretory granules, but less than 1% of these are thought to be available for immediate release [71]. All the rest are considered to be immature, Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 13 and must be primed and then recruited to the cell membrane before they can undergo exocytosis. These processes require several ATP-, Ca2+-, and phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent steps, which culminate in pore formation and granule release to the cell exterior [70, 76]. 2.1 Before proteomics: major protein components of the β-cell secretory granule The protein composition of the endocrine pancreas has arguably been under investigation for most of the past 100 years. The initial impetus was provided by the search for the hypoglycaemic principle, which turned out to be insulin [25]. For many years after its discovery, until the advent of recombinant human insulin manufactured in microbial expression systems, bovine and porcine pancreases were the only feasible sources of insulin for clinical use [77]. The extraction of pharmacologically-active insulin from the pancreas, wherein the predominant exocrine cells are replete with proteolytic hormones, was said to be one of the most challenging of all extractions of natural products for pharmaceutical purposes [77]. The considerable heterogeneity of highly-purified insulin preparations was demonstrated by application of various chromatographic and electrophoretic methods well before the discovery of proinsulin. Biochemical analysis of the pancreatic islets subsequently yielded the insulinprecursor, proinsulin [42, 78, 79], and with it in time the understanding of insulin release by enzyme-catalysed conversion from proinsulin [42]. These pre-proteomic era studies provided a platform on which current proteomic analysis of the β-cell secretory granule may be anchored. Modern proteomic studies are thus seen as an extension of this earlier work. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 14 (Figure 3 near here) 3. Evolution of a question that might be addressed by proteomics: “How might amylin misfolding cause T2DM?” Islet amyloid is formed mainly by misfolded human amylin [27], a physiological resident of the islet β-cell granule (Figures 1, 3). Aggregation of the human hormone into small soluble β-sheetcontaining oligomers is linked to islet β-cell degeneration and the pathogenesis of T2DM [3133]. Islet amyloid is associated with substantial reductions in relative β-cell mass in type-2 diabetes (on average ~60 %), probably due to increased apoptosis compared with obese and lean non-diabetic humans [80]. Several lines of evidence now provide compelling support for the idea that processes associated with amylin aggregation contribute to β-cell degeneration and the onset of T2DM. First, in vitro studies with synthetic amylin show that fibrillar structures assemble spontaneously through self-association of monomers into protofibrils and higher-order fibrillar structures [81, 82]. Studies with time-dependent atomic force microscopy have enabled direct in vitro visualization of this process. These studies show that oligomer formation can take place within minutes [31], a time-course that matches the activation of the β-cell membrane Fas/FasL/FADD-activated pathway in β-cells destined to undergo amylin-evoked apoptosis [33]. Cytotoxic amylin preparations contain few preformed fibrils, but undergo time-dependent aggregation into soluble β-conformers [83]. Islet β-cell toxicity evoked by aggregating extracellular amylin, occurs through an apoptotic mechanism [84, 85] mediated via a pathway Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 15 comprising initial activation of a membrane-bound Fas/FasL/FADD/caspase-8 complex [33, 86] followed by a three-pronged downstream cascade comprising JNK1/cJun [86], ATF2/p38 MAPK [87], and p53/p21WAF1/CIP1 [85], that results ultimately in activation of caspase-3 [86] and consequent apoptosis. In addition, parallel amylin-mediated activation of ER-stress related pathways might also contribute to islet β-cell degeneration [88]. Second, associations between human amylin aggregation and decreased β-cell mass have been reported from in vivo studies in several murine transgenic models of amylin-mediated diabetes [29, 32, 89-93] (Figure 5). Similar associations are present in primates, whose wild-type amylin molecules contain an amyloidogenic sequence [24, 94, 95], are fibrillogenic, and form islet amyloid [96-99]. By contrast, murine amylin molecules are not aggregation-prone [100], so diabetic phenotypes in human amylin transgenic mice develop in a background devoid of amyloid formed by the wild-type murine hormone. Obese human amylin-transgenic mice have been reported to replicate pathological findings in T2DM, showing non-ketotic hyperglycaemia, amyloid deposition, and decreased β-cell mass, possibly via increased apoptosis [92]. (Figure 5 near here) Amylin aggregation could thus mediate β-cell degeneration in T2DM. However, the significance of tissue aggregates comprising mature amyloid fibrils in T2DM pathogenesis remains uncertain, since some studies have implied that amyloid fibrils themselves may be toxic, or that human amylin-transgenic mice may not develop spontaneous diabetes [91, 101-104]. The latter discrepancies may well be explained however, by a requirement for permissive genetic background-human amylin transgene interactions to manifest full-blown β-cell degeneration and a diabetic phenotype [32, 93, 105]. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 16 Finally, human amylin purified from islet amyloid deposits is a potent inducer of insulin resistance in ex vivo rat skeletal muscle [106, 107] (Figure 5). This finding provides a potential link between islet β-cell dysfunction and the induction of peripheral insulin resistance. (Figure 6 near here) These observations, and the ongoing uncertainty concerning precise mechanistic linkages between amylin aggregation, β-cell degeneration, the regulation of systemic fuel metabolism, and T2DM onset [108], provide fertile ground for future proteomic investigation. For example, the exact location and mechanism of amylin misfolding is unknown, as is the site of origin and nature of the amylin-mediated death-initiating signal – is it cell membrane-bound Fas/FasL/FADD activation [33], ER stress [88], or some other process that might occur elsewhere [109, 110]? One key question that is yet to be answered, is whether amylin-mediated misfolding occurs prior to, within, or after amylin secretion from the pancreatic islet β-cell granule. Granulefocussed studies are expected to prove crucial in answering this key question. 4. The β-cell secretory granule proteome Available proteomic studies of whole mouse [111] and human [112] islets have identified groups of islet-specific proteins. The bulk of proteins detected overlap with those in other tissue types, but islet hormones were also identified. The resulting peptide reference libraries are seen as providing a resource for future higher-throughput and quantitative studies of islet biology, which may be useful in the study of T2DM mechanisms, for example. Proteomics has been applied to analyse the effects of insulin signalling in isolated murine and human islets, in a study that provides a useful example of how this methodology can be Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 17 integrated with other methods to address a complex research question – in this case, the role of insulin in β-cell apoptosis [113]. In this study, combined data, to which proteomic analysis made a significant contribution, indicated that insulin can act as a master regulator of islet survival by regulating Pdx1. 4.1 Proteomic approach to the β-cell secretory granule Two systematic proteomic analyses of βcell secretory granules have been reported in the past few years [20, 22]. Results from one of these are detailed here (Table 1) as a basis for the following comparisons and contrasts [20]. (Table 1 near here) These studies reported the molecular identities of 51 and 130 ‘granule-related’ proteins [20, 22], respectively. In each case, most of the proteins reported were newly identified as potential granule components. Without doubt, these findings usher in a new era of studies of the β-cell secretory granule. These studies point to numerous proteins and pathways that have not previously been identified in β-cell secretory granules, and thus have the potential to greatly increase our knowledge of the intricate functionality of these organelles. However, comparisons and contrasts between the two are illustrative of some of the challenges confronted by application of proteomics to the β-cell secretory granule at present, and caution is warranted in their interpretation and application. Intensive follow-up work is required before the status of each newly-identified putative granule protein is confirmed and clarified. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 18 Both groups analysed granules purified from insulin-secreting rat INS-1E cells, which came initially from the same source. Comparisons and contrasts between the two studies are therefore informative for several reasons, so they are now described in greater detail. 4.2 Method comparisons Both studies identified many proteins that had previously been associated with β-cell secretory granules by pre-proteomic methods, and there were considerable overlaps between the two data sets [20, 22]. However, Brunner et al reported many proteins usually considered to be lysosomeassociated, as components of their purified β-cell secretory granule proteome. By contrast, Hickey et al described numbers of proteins more usually associated with ER and mitochondria, some of which did not appear in the data set of Brunner et al. These differences deserve further consideration, since they highlight potential challenges faced by current proteomic techniques. In order to focus exclusively on β-cell secretory granule-associated proteins, both groups employed several purification steps prior to protein identification analysis. These studies illustrate the principle that increased sensitivity and specificity can potentially be achieved by prior organellar purification. Brunner et al employed two sequential ultracentrifugation steps: a first-dimensional layered-discontinuous Nycodenz gradient followed by a Percoll cushion [22]. They monitored protein recovery by using an insulin immunoassay (granule marker) and western blotting for calreticulin and betagranin, respectively as markers for ER and β-cell secretory granules . They then separated proteins by using one-dimensional SDS-PAGE and sectioning gels into 28 consecutive pieces, followed by in-gel tryptic digestion and protein identification analysis using Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 19 LC-MS2 followed by bioinformatic methods. They also monitored relative purity of granule preparations by western blotting for a series of markers associated with various extra-granular organelles. These findings pointed to the presence of lysosomes within their granule preparations, so the significance of the putative assignments of lysosomal proteins as components of the granule proteome, remains to be confirmed. By contrast, Hickey et al chose to isolate β-cell secretory granules using a conservative methodology that initially employed two sequential orthogonal steps with concomitant monitoring for several, potentially-confounding organelles including lysosomes, ER, mitochondria and cytosol [20]. The first of these used ultracentrifugation after overlayering INS1E culture supernatants onto preformed, continuous OptiPrep gradients with subsequent fractionation and separation of granule-containing fractions, as determined by their hormone content [114], whose buoyant density was 1.10-1.11. They monitored this step using an insulin immunoassay as a granule marker coupled with multiple enzyme assays to track potential contamination with other organelles, including aryl sulphatase (lysosomal marker), NADH cytochrome C reductase (ER), citrate synthase (mitochondria), and lactate dehydrogenase (cytosol) (Figure 7). (Figure 7 near here) In the following, orthogonal step, they immunopurified granules by using anti-VAMP2 antibody-conjugated magnetic Dynal beads [20], and then undertook protein identification analysis that comprised the following sequential steps: reduction and S-carboxymethylation; snap-freezing and concentration by vacuum centrifugation; trypsinization; peptide recovery by Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 20 batch reversed-phase chromatography; and LC-MS2 with stringent bioinformatic criteria for inclusion in the final list. 4.3 How to account for contrasting conclusions? Although there are areas of substantive overlap in the results and conclusions, there were also broad areas of divergence between these two studies [20, 22]. Why might this be so? Both groups went to considerable lengths to ensure the purity of the granular preparations, whose contents they subsequently analysed. Hickey et al assigned proteins to specific sub-cellular locations or functions by application of currently-accepted rules. By contrast, Brunner et al simply listed numerous mitochondrial proteins under the category of ‘Other Proteins’ (for example: 10-kDa heat shock protein; mitochondrial cystatin-C precursor; malate dehydrogenase, mitochondrial precursor; superoxide dismutase (whether this was SOD1 or SOD 2 they did not specify – there is evidence from others that both forms localize in mitochondria); voltage-dependent anion-selective channel protein 1 (VDAC); and ATP synthase alpha chain, mitochondrial precursor), without allocating proteins to specific locations or tasks. Their findings with respect to this sub-group of proteins were generally consistent with those of Hickey et al, who also reported significant numbers of mitochondria-associated proteins in their granule preparations. Brunner et al did not specify NCBI accession numbers for their individual proteins, so the exact molecular identity of some, for example, ‘superoxide dismutase’, remains uncertain. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 21 Brunner et al. also reported an abundance of lysosomal proteins, which were annotated as hydrolases or lysosomal membrane proteins [22]. However, by contrast, none of these was detected by Hickey et al [20]. 4.4 Possible explanations for between-study divergence Why were there such major differences between the two reports? Clearly, the two studies employed different isolation methods, so it was possible that there would be some differences between the sets of proteins identified. However, the divergences between large groups of proteins with shared prior subcellular localizations, indicates that more systematic factors were probably also at work. The lack of lysosomeassociated proteins in the second study, might reflect the use of enzyme markers during the fractionation which, when coupled with the following orthogonal immunopurification, permitted exclusion of lysosome-rich fractions from the final preparation. In the reverse direction, 20% of the proteins identified by Hickey et al were chaperones, whose main agreed functions reside in the ER, and a further 20% comprised other proteins with known ER- or Golgi-related functions (Figure 8). Clearly, the existence of a large number of chaperone-related proteins in the β-cell secretory granule, if confirmed, could have important implications for disorders triggered by protein misfolding, such as aggregation-associated β-cell degeneration [32]. (Figure 8 near here) However, the conclusions from these two preliminary forays into the field of β-cell secretory granule proteomics may best be viewed as providing the basis for further, intensive research. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 22 The immunoaffinity purification procedures employed by Hickey et al may have purified not only granules, but also aspects of the cytoskeletal apparatus responsible for granule transport, which therefore may adhere to them (for example, actin, tubulin, and Rab GTP-binding proteins). Similarly, the presence of ER and cytosolic components in those results may follow from intracellular associations between secretory granules and aspects of the associated structures. As one example, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), which was also detected in that study, has several cellular roles and locations, and is known to bind to microtubules [115]. However, there is evidence that attachments between secretory granules and components of the ER and cytoskeleton are physiological, so the findings of ER- and cytoskeleton-related proteins in purified granule preparations may well reflect these associations. 4.5 Calreticulin: putative assignment as an insulin-granule protein or indicator of ER-protein contamination? Hickey et al identified calreticulin in their β-cell secretory granule isolates [20], whereas Brunner et al specifically excluded it from their ‘immature secretory granule (ISG)’ preparations [22]. The latter findings can be interpreted to indicate that the former preparations were contaminated with ER-derived proteins. Alternatively, calreticulin might be associated with a subset of granules (ISGs) not purified in Brunner et al’s approach. What does the balance of currently-available evidence suggest concerning this interesting and potentially significant difference? There are reports that calreticulin fulfils distinct roles in different organelles. It has three functional domains (termed N-, P-, and C-). The first binds KXFFKR motifs, thereby acting as a chaperone [116], and also binds Zn2+ [117], which is noted Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 23 to be present at high concentrations in the granule core. The latter two domains contain Ca2+binding sites and may thus function as local Ca2+ stores [118]. Although a COOH-terminal KDEL sequence could be seen as consistent with specific targetting to the ER, there is available evidence that calreticulin also localizes to cytoplasmic granules in T-lymphocytes [117] and sperm acrosomes, and to the Golgi complex [119], and that it is systemically secreted to circulate in the plasma [120]. B-cell secretory granules contain a high-Ca2+ environment necessary for proteolytic processing [64, 73, 121, 122], so Hickey et al’s findings could also be consistent with a role for calreticulin as a newly-recognised Ca2+-storage protein in β-cell secretory granules. The preceding analysis points to a series of specific and testable hypotheses. Evidently, an early consideration is that assignment of calreticulin as a putative insulin-granule protein requires independent confirmation. 4.6 How might the appearance of ‘mitochondrial proteins’ in ‘insulin-granule-specific’ preparations be interpreted? The presence in insulin-granule preparations of proteins usually associated with mitochondria requires comment. It is now becoming evident that apparently discrete organelles do not necessarily contain discrete protein sets. Interestingly, ‘organelle-specific’ proteomic analyses have frequently demonstrated multiple locations for up to perhaps 40% of all component proteins [123]. Therefore, many proteins, which until now have not been associated with granules, may in fact be localized in or adherent to them, and thus serve roles in granule function additional to those already assigned to them in other organelles. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 24 For example, both studies [20, 22] detected component chains of the mitochondrial ATP synthase in purified granules, whereas until recently these subunits have mainly been considered to play roles in oxidative phosphorylation. However, the α-chain of ATP-synthase was recently shown to act as a receptor for apolipoprotein A-1-mediated hepatic LDL endocytosis [124]. Thus, the current findings might signal that ATP-synthase chains play further, hitherto unsuspected roles in β-cell secretory granule homeostasis. A further explanation for the presence of a number of ‘mitochondrial proteins’ in β-cell insulin-granule preparations is that of the physical attachment between the two organelles that causes their obligatory co-purification. There is evidence that mitochondria are attached to cytoskeletal structures in hepatocytes, ciliary desmosomes [125], PC12 cells [126], and neurosecretory vesicles [127], and to pancreatic islet β-cell granules [62]. The proton-pumping requirements for granule maturation [128], within-granule chaperone activity, and insulin exocytosis are dependent on mitochondria-derived ATP [126]. Furthermore, mutations in mitochondrial DNA can cause β-cell dysfunction and rare forms of T2DM [129]. The close proximity of mitochondria to granules could enhance efficient transfer of ATP to drive granuleassociated processes, for example their intracellular translocation, and within-granule protein processing, maturation and exocytosis. 4.7 Chaperones in the β-cell secretory granule: possible implications Aggregation of human amylin into β-sheet-containing oligomers has been linked to islet β-cell dysfunction and the causation of the common form of T2DM [32, 33, 93]. The existence of amylin misfolding in T2DM [108], points to a defect in regulation of the β-cell protein-folding pathways as a key target in the search for the underlying cause of islet β-cell dysfunction. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 25 Within this context, the identification of specific chaperones in the β-cell granule provides a clear mechanistic linkage between protein misfolding in diabetes and a newlyemergent, putative granule function. For example, alterations in the function of specific chaperones and their interactions with amylin can now be investigated as potential contributors to amylin misfolding. These findings represent a major potential contribution of proteomic analysis to the search for the molecular basis of disease mechanisms in diabetes. In the study of Hickey et al, ER- and Golgi-associated proteins accounted for almost 40% of all those identified (Table 1), consistent with close association between β-cell secretory granules , and elements of the ER and Golgi apparatus. Particularly abundant in these preparations were recognised chaperone proteins, which comprised ~20% of all identified proteins in their preparations [20]. These data are consistent with the idea that β-cells may act as a significant source of secreted/circulating chaperone proteins. The localization of chaperones in β-cell granules might be considered unremarkable, given that they are abundant in most tissues and organelles. Consistent with these data, others have reported that HSPs and other chaperone proteins are plentiful in pancreatic islets [17, 130], where some are co-localized in secretory granules [56, 57, 131] or synaptophysin-containing microvesicles [132]. HSPs are also localized in the extracellular compartment [133] and their serum concentrations can vary according to physiological status [134]. Different chaperone molecules act cooperatively [134], consistent with the observed diversity in the β-cell secretory granules . HSP90 localizes in neuronal tissues [135], and a recent subcellular proteomic dissection of neuromelanin granules also reported high levels of ERderived chaperones [136], consistent with its presence in pancreatic β-cells. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 26 The apparent abundance of chaperones in β-cell secretory granules highlights their likely roles in preserving intact and well-regulated secretion of the hormones, amylin and insulin, and protecting the cell from the biological effects of protein misfolding [20, 32, 33]. The amylingenerated aggregates present in T2DM [27, 108] are similar to those that occur in Alzheimer’s, Parkinson’s and Huntington’s diseases [137]. At high concentrations, dysregulated amylin folding occurs independently of protein convertases (PC2, or PC1/3), to potentially seed the amyloid fibrils that in turn form the tissue aggregates designated as ‘islet amyloid’ [81, 100, 138]. There is no direct evidence, however, for the presence of aberrantly-processed human amylin in pancreatic islet amyloid [108]. Further proteomic analysis will be required to determine whether aberrant amylin processing is a contributor to islet amyloidosis and the origins of T2DM. During its transition from soluble oligomers through protofibrils to insoluble fibrils [30, 31, 81, 100, 139], misfolding human amylin can elicit cytotoxic processes that can cause or result in β-cell death [83, 139]. This process in turn can cause decreased pancreatic β-cell mass and insulin secretory capacity, resulting in hyperglycaemia and, ultimately, T2DM [32, 33, 93, 140]. It may also serve as a target in the development of new classes of anti-diabetic compounds [32, 141]. 5. Next steps This chapter has presented reasons why it is desirable to undertake further detailed proteomic studies of the islet β-cell secretory granule. In particular, it is expected that important insights concerning the physiological regulation of islet hormone secretion and β-cell death, and the origins and mechanisms of T1DM and T2DM, might be obtained through such analysis. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 27 However, the significant discrepancies between the two data sets discussed above [20, 22], show that currently-available approaches have clear limitations. In particular, it is apparent that each new protein assignment to the islet β-cell granule proteome must be regarded as no more than putative until substantive confirmatory evidence is obtained, by application of more selective proteomic analysis as well as one or more orthogonal methods, such as immunological co-localization of proteins to the granule. Results from even the latter must be viewed with caution, however, since limits of sensitivity and specificity of individual antibodies may provide limitations that must be taken into account. At present, there are no available proteomic studies of β-cell granules from diabetic humans, so inferences concerning diabetes-related changes in granule composition, and how such effects might contribute to the phenomena of diabetic β-cell degeneration must be drawn from other lines of investigation. There is a clear need for more precise information to characterise the changes in islet structure and function that occur in relation to the origins of βcell dysfunction, islet amyloid formation and β-cell disappearance in T2DM. It is to be hoped that the ongoing rapid development of new and improved proteomic approaches will enable such studies in the near future. Indeed, it is expected that one of the exciting outcomes of the current assembly of expert views in this volume will be the creation of linkages and methodologies by which these important goals can be facilitated. Acknowledgements I wish to acknowledge my wife, Margaret Cameron-Cooper, for her unswerving loyalty and steadfast support throughout the past 30 years of scientific endeavour; Professor Sir P John Scott for his guidance and wise counsel; Cynthia Tse for her valuable help Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 28 with preparation of the manuscript; and all my scientific colleagues, who have contributed to our group’s studies in so many different ways. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 29 6. References [1] Jüllig M, Hickey AR, Chai CC, Skea GL, Middleditch MJ, Costa S, et al. Is the failing heart out of fuel or a worn engine running rich? A study of mitochondria in old spontaneously hypertensive rats. Proteomics 2008;8:2556-72. [2] Wang Y, Xu A, Knight C, Xu LY, Cooper GJS. Hydroxylation and glycosylation of the four conserved lysine residues in the collagenous domain of adiponectin: potential role in the modulation of its insulin-sensitizing activity. J Biol Chem 2002;277:19521-9. [3] Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nature Biotechnol 2003; 21:255-61. [4] Atkinson KR, Blumenstein M, Black MA, Wu SH, Kasabov N, Taylor RS, et al. An altered pattern of circulating apolipoprotein E3 isoforms is implicated in preeclampsia. J Lipid Res 2009;50:71-80. [5] Savitski MM, Nielsen ML, Zubarev RA. ModifiComb, a new proteomic tool for mapping substoichiometric post-translational modifications, finding novel types of modifications, and fingerprinting complex protein mixtures. Mol Cell Proteomics 2006;5:935-48. [6] Syka JEP, Coon JJ, Schroeder MJ, Shabanowitz J, Hunt DF. Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc Natl Acad Sci USA 2004;101:9528-33. [7] Wang Y, Lam KS, Chan L, Chan KW, Lam JB, Lam MC, et al. Post-translational modifications of the four conserved lysine residues within the collagenous domain of adiponectin are required for the formation of its high molecular weight oligomeric complex. J Biol Chem 2006;281:16391-400. [8] Wilkins MR, Gasteiger E, Gooley AA, Herbert BR, Molloy MP, Binz PA, et al. Highthroughput mass spectrometric discovery of protein post-translational modifications. J Mol Biol 1999;289:645-57. [9] Blumenstein M, McMaster MT, Black MA, Wu S, Prakash R, Cooney J, et al. A proteomic approach identifies early pregnancy biomarkers for preeclampsia: novel linkages between a predisposition to preeclampsia and cardiovascular disease. Proteomics 2009;9:2929-45. [10] Wang Y, Xu A, Ye JM, Kraegen EW, Tse CA, Cooper GJS. Altered phosphorylation of P20 occurs in diabetic rats with insulin resistance. Diabetes 2001;50:1821-7. [11] Blumenstein M, Prakash R, Cooper GJS, North RA, on behalf of the SCOPE consortium. Aberrant processing of plasma vitronectin and high molecular weight kininogen precedes the onset of preeclampsia. Reprod Sci 2009;16:1144-52. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 30 [12] Jüllig M, Chen X, Hickey AJ, Crossman DJ, Xu A, Wang Y, et al. Reversal of diabetesevoked changes in mitochondrial protein expression of cardiac left ventricle by treatment with a copper(II)-selective chelator. Proteomics Clin Appl 2007;1:387-99. [13] Pierce A, Unwin RD, Evans CA, Griffiths S, Carney L, Zhang L, et al. Eight channel iTRAQ enables comparison of the activity of 6 leukaemogenic tyrosine kinases. Mol Cell Proteomics 2008;7:853-63. [14] Jefferson LS, Cherrington AD, (Eds.). The Endocrine Pancreas and Regulation of Metabolism. New York, N. Y.: American Physiological Society, Oxford University Press, 2001. [15] Guest PC, Bailyes EM, Rutherford NG, Hutton JC. Insulin secretory granule biogenesis: coordinate regulation of the biosynthesis of the majority of constituent proteins. Biochem J 1991;274:73-8. [16] Hu L, Evers S, Lu ZH, Shen Y, Chen J. Two-dimensional protein database of human pancreas. Electrophoresis 2004;25:512-8. [17] Nicolls MR, D’Antonio JM, Hutton JC, Gill RG, Czwornog JL, Duncan MW. Proteomics as a tool for discovery: proteins implicated in Alzheimer’s disease are highly expressed in normal pancreatic islets. J Proteome Res 2003;2:199-205. [18] Sparre T, Larsen MR, Heding PE, Karlsen AE, Jensen ON, Pociot F. Unravelling the pathogenesis of type 1 diabetes with proteomics: present and future directions. Mol Cell Biol 2005;4:441-57. [19] Metz TO, Jacobs JM, Gritsenko MA, Fontes G, Qian WJ, Camp DG, et al. Characterization of the human pancreatic islet proteome by two-dimensional LC/MS/MS. J Proteome Res 2006;5:3345-54. [20] Hickey AJ, Bradley JW, Skea GL, Middleditch MJ, Buchanan CM, Phillips AR, et al. Proteins associated with immunopurified granules from a model pancreatic islet beta-cell system: proteomic snapshot of an endocrine secretory granule. J Proteome Res 2009;8:178-86. [21] Mittal A, Phillips AR, Middleditch MJ, Ruggiero K, Loveday B, Delahunt B, et al. The proteome of mesenteric lymph during acute pancreatitis and implications for treatment. J Pancreas 2009;10:130-42. [22] Brunner Y, Couté Y, Iezzi M, Foti M, Fukuda M, Hochstrasser DF, et al. Proteomic analysis of insulin secretory granules. Mol Cell Proteomics 2007;6:1007-17. [23] Opie EL. The relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of the islands of Langerhans. J Exp Med 1900-01;5:527-40. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 31 [24] Cooper GJS. Amylin compared with calcitonin gene-related peptide: structure, biology, and relevance to metabolic disease. Endocr Rev 1994;15:163-201. [25] Banting FG, Best CH, Collip JB, Campbell WR, Fletcher AA. Pancreatic extracts in the treatment of diabetes mellitus. Preliminary report. Can Med Assoc J 1922;12:141-6. [26] Aanstoot HJ, Kang SM, Kim J, Lindsay L, Roll U, Knip M, et al. Identification and characterization of Glima 38, a glycosylated islet cell membrane antigen, which together with GAD 65 and IA2, marks the early phases of autoimmune response in type 1 diabetes. J Clin Invest 1996;97:2772-83. [27] Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci USA 1987;84:8628-32. [28] Höppener JW, Ahrén B, Lips CJ. Islet amyloid and type 2 diabetes mellitus. N Engl J Med 2000;343:411-9. [29] Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. β-cell deficit and increased β-cell apoptosis in humans with type 2 diabetes. Diabetes 2003;52:102-10. [30] Goldsbury C, Kistler J, Aebi U, Arvinte T, Cooper GJS. Watching amyloid fibrils grow by time lapse atomic force microscopy. J Mol Biol 1999;285:33-9. [31] Green JD, Goldsbury C, Kistler J, Cooper GJS, Aebi U. Human amylin oligomer growth and fibril elongation define two distinct phases in amyloid formation. J Biol Chem 2004;279:12206-12. [32] Aitken JF, Loomes KM, Scott DW, Reddy S, Phillips ARJ, Prijic G, et al. Tetracycline treatment retards the onset and slows the progression of diabetes in human amylin transgenic mice. Diabetes 2010;59:161-71. [33] Zhang S, Liu H, Yu H, Cooper GJS. Fas-associated Death Receptor signaling evoked by human amylin in islet β-cells. Diabetes 2008;57:348-56. [34] Elias D, Markovits D, Reshef T, Van der Zeet R, Cohen IR. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci USA 1990;87:1576-80. [35] Atkinson MA, Maclaren NK. Islet cell autoantigens in insulin-dependent diabetes. J Clin Invest 1992;92:1608-16. [36] Brudzynski K, Martinez V, Gupta RS. Secretory granule autoantigen in insulindependent diabetes mellitus is related to 62 kDa heat-shock protein (hsp60). J Autoimmun 1992;5:453-63. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 32 [37] Solimena M, Dirk Jr R, Hermel JM, Pleasic-Williams S, Shapiro JA, Caron L, et al. ICA 512, an autoantigen of type I diabetes, is an intrinsic membrane protein of neurosecretory granules. EMBO J 1996;15:2102-14. [38] Roep BO. T-cell responses to autoantigens in IDDM. The search for the Holy Grail. Diabetes 1996;45:1147-56. [39] Tree TIM, O'Byrne D, Tremble JM, MacFarlane WM, Haskins K, James RFL, et al. Evidence for recognition of novel islet T cell antigens by granule-specific T cell lines from new onset type 1 diabetic patients. Clin Exp Immunol 2000;121:100-5. [40] Docherty K, Hutton JC, Steiner DF. Cathepsin B-related proteases in the insulin secretory granule. J Biol Chem 1984;259:6041-4. [41] Hutton JC. Insulin secretory granule biogenesis and the proinsulin-processing endopeptidases. Diabetologia 1994;37 Suppl 2:S48-56. [42] Steiner DF, Park SY, Støy J, Philipson LH, Bell GI. A brief perspective on insulin production. Diabetes Obes Metab 2009;11 Suppl 4:189-96. [43] Wang WY, Barratt BJ, Clayton DG, Todd JA. Genome-wide association studies: theoretical and practical concerns. Nat Rev Genet 2005;6:109-18. [44] Hamming KSC, Soliman D, Matemisz LC, Niazi O, Lang Y, Gloyn AL, et al. Coexpression of the type 2 diabetes susceptibility gene variants KCNJ11 E23K and ABCC8 S1369A alter the ATP and sulfonylurea sensitivities of the ATP-sensitive K+ channel. Diabetes 2009;58:2419-24. [45] Jonsson A, Isomaa B, Tuomi T, Taneera J, Salehi A, Nilsson P, et al. A variant in the KCNQ1 gene predicts future type 2 diabetes and mediates impaired insulin secretion. Diabetes 2009;58:2409-13. [46] Ashcroft FM, Harrison ED, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature 1984;312:446-8. [47] Ashcroft FM. ATP-sensitive potassium channelopathies: focus on insulin secretion. J Clin Invest 2005;115:2047-58. [48] Braun M, Ramracheya R, Bengtsson M, Zhang Q, Karanauskaite J, Partridge C, et al. Voltage-gated ion channels in human pancreatic beta-cells: electrophysiological characterization and role in insulin secretion. Diabetes 2008;57:1618-28. [49] Sheu L, Pasyk EA, Ji J, Huang X, Gao X, Varoqueaux F, et al. Regulation of insulin exocytosis by Munc13-1. J Biol Chem 2003;278:27556-63. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 33 [50] Cheviet S, Coppola T, Haynes LP, Burgogyne RD, Regazzi R. The Rab-binding protein Noc2 is associated with insulin-containing secretory granules and is essential for pancreatic β-cell exocytosis. Mol Endocrinol 2004;18:117-26. [51] Yaekura K, Julyan R, Wicksteed BL, Hays LB, Alarcon C, Sommers S, et al. Insulin secretory deficiency and glucose intolerance in Rab3A Null mice. J Biol Chem 2003;278:9715-21. [52] Yi Z, Yokota H, Torii S, Aoki T, Hosaka M, Zhao S, et al. The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules. Mol Cell Biol 2002; 22:1858-67. [53] Iezzi M, Regazzi R, Wollheim CB. The Rab3-interacting molecule RIM is expressed in pancreatic β-cells and is implicated in insulin exocytosis. FEBS Lett 2000;474:66-70. [54] Coppola T, Frantz C, Perret-Menoud V, Gattesco S, Hirling H, Regazzi R. Pancreatic βcell protein granuphilin binds Rab3 and Munc-18 and controls exocytosis. Mol Biol Cell 2002;13:1906-15. [55] Varadi A, Ainskow EK, Allan VJ, Rutter GA. Involvement of conventional kinesin in glucose-stimulated secretory granule movements and exocytosis in clonal pancreatic beta-cells. J Cell Sci 2002;115:4177-89. [56] Brown H, Larsson O, Bränström R, Yang SN, Leibiger B, Leibiger I, et al. Cysteine string protein (CSP) is an insulin secretory granule-associated protein regulating beta-cell exocytosis. EMBO J 1998;17:5048-58. [57] Zhang H, Kelley WL, Chamberlain LH, Burgoyne RD, Lang J. Mutational analysis of cysteine-string protein function in insulin exocytosis. J Cell Sci 1999;112:1345-51. [58] Wasmeier C, Hutton JC. Secretagogue-dependent phosphorylation of the insulin granule membrane protein phogrin is mediated by cAMP-dependent protein kinase. J Biol Chem 2001;276:31919-28. [59] Iezzi M, Escher G, Meda P, Charollais A, Baldini G, Darchen F, et al. Subcellular distribution and function of Rab3A, B, C, and D isoforms in insulin-secreting cells. Mol Endocrinol 1999;13:202-12. [60] Regazzi R, Sadoul K, Meda. P., KeIly RB, Halban PA, Wollheim CB. Mutational analysis of VAMP domains implicated in Ca2-induced insulin exocytosis. EMBO J 1996;15:6951-9. [61] Masgrau R, Churchill GC, Morgan AJ, Ashcroft SJ, Galione A. NAADP: a new second messenger for glucose-induced Ca2 responses in clonal pancreatic beta cells. Curr Biol 2003;13:247-51. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 34 [62] Mitchell KJ, Lai FA, Rutter GA. Ryanodine receptor type I and nicotinic acid adenine dinucleotide phosphate receptors mediate Ca2 release from insulin-containing vesicles in living pancreatic β-cells (MIN6). J Biol Chem 2009;278:11057-64. [63] Lee HC, Aarhus R. A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J Biol Chem 1995;270:2152-7. [64] Galione A, Evans AM, Ma J, Parrington J, Arredouani A, Cheng X, et al. The acid test: the discovery of two-pore channels (TPCs) as NAADP-gated endolysosomal Ca2 release channels. Pflügers Arch 2009;458:869-76. [65] Patel S, Churchill GC, Galione A. Coordination of Ca2 signalling by NAADP. Trends Biochem Sci 2001;26:482-9. [66] Johnson JD, Misler S. Nicotinic acid-adenine dinucleotide phosphate-sensitive calcium stores initiate insulin signaling in human beta cells. Proc Natl Acad Sci USA 2002;99:14566-71. [67] Orci L. The insulin factory: a tour of the plant surroundings and a visit to the assembly line. The Minkowski lecture 1973 revisited. Diabetologia 1985;28:528-46. [68] Hutton JC. The insulin secretory granule. Diabetologia 1989;32:271-81. [69] Hutton J, Penn E, Peshavaria M. Low-molecular-weight constituents of isolated insulinsecretory granules: bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J 1983;210:297-305. [70] Takahashi N, Kishimoto T, Nemoto T, Kadowaki T, Kasai H. Fusion pore dynamics and insulin granule exocytosis in the pancreatic islet. Science 2002;297:1349-52. [71] Rorsman P, Renstrom E. Insulin granule dynamics in pancreatic beta cells. Diabetologia 2003;46:1029-45. [72] Howell SL, Young DA, Lacy PE. Isolation and properties of secretory granules from rat islets of Langerhans. III. Studies on the stability of the isolated beta granules. J Cell Biol 1969;41:167-76. [73] Hutton JC, Penn E, Peshavaria M. Isolation and characterisation of insulin secretory granules from a rat islet cell tumour. Diabetologia 1982;2:365-73. [74] Sopwith AM, Hales DF, Hutton JC. Pancreatic B-cells secrete a range of novel peptides besides insulin. Biochim Biophys Acta 1984;803:342–5. [75] Westermark P, Wernstedt C, Wilander E, Hayden DW, O’Brien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A 1987;84:3881-5. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 35 [76] Nagamatsu S, Ohara-Imaizumi M, Nakamichi Y, Kikuta T, Nishiwaki C. Imaging docking and fusion of insulin granules induced by antidiabetes agents. Sulfonylurea and glinide drugs preferentially mediate the fusion of newcomer, but not previously docked, insulin granules. Diabetes 2006;55:2819-25. [77] Burgermeister W, Enzmann F, Schöne HH. The Isolation of Insulin from the Pancreas. Insulin: Handbook of Experimental Pharmacology XXXII/2. Berlin: Springer-Verlag, 1975. p. 715-27. [78] Grant PT, Reid KB. Biosynthesis of an insulin precursor by islet tissue of cod (Gadus callarias). Biochem J 1968;110:281-8. [79] Steiner DF, Cunningham D, Spigelman L, Aten B. Insulin biosynthesis: evidence for a precursor. Science 1967;157:697-700. [80] Zhao HL, Lai FM, Tong PC, Zhong DR, Yang D, Tomlinson B, et al. Prevalence and clinicopathological characteristics of islet amyloid in Chinese patients with type 2 diabetes. Diabetes 2003;52:2759-66. [81] Goldsbury CS, Cooper GJS, Goldie KN, Mueller SA, Saafi EL, Gruijters WTM, et al. Polymorphic fibrillar assembly of human amylin. J Struct Biol 1997;119:17-27. [82] Goldsbury C, Goldie K, Pellaud J, Seelig J, Frey P, Muller SA, et al. Amyloid fibril formation from full-length and fragments of amylin. J Struct Biol 2000;130:352-62. [83] Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJS. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J 2006;273:3614-24. [84] Janciauskiene S, Ahrén B. Different sensitivity to the cytotoxic action of IAPP fibrils in two insulin-producing cell lines, HIT-T15 and RINm5F cells. Biochem Biophys Res Commun 1998;251:888-93. [85] Zhang S, Liu J, Saafi EL, Cooper GJS. Induction of apoptosis by human amylin in RINm5F islet β-cells is associated with enhanced expression of p53 and p21WAF1/CIP1. FEBS Lett 1999;455:315-20. [86] Zhang S, Liu J, Dragunow M, Cooper GJS. Fibrillogenic amylin evokes islet β-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem 2003;278:52810-9. [87] Zhang S, Liu H, Liu J, Tse CA, Dragunow M, Cooper GJS. Activation of activating transcription factor 2 by p38 MAP kinase during apoptosis induced by human amylin in cultured pancreatic β-cells. FEBS J 2006;273:3779-91. [88] Haataja L, Gurlo T, Huang CJ, Butler PC. Islet amyloid in type 2 diabetes, and the toxic oligomer hypothesis. Endocr Rev 2008;29:303-16. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 36 [89] Janson J, Soeller WC, Roche PC, Nelson RT, Torchia AJ, Kreutter DK, et al. Spontaneous diabetes mellitus in transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci USA 1996;93:7283-8. [90] Soeller WC, Janson J, Hart SE, Parker JC, Carty MD, Stevenson RW, et al. Islet amyloid-associated diabetes in obese A(vy)/a mice expressing human islet amyloid polypeptide. Diabetes 1998;47:743-50. [91] Höppener JW, Oosterwijk C, Nieuwenhuis MG, Posthuma G, Thijssen JH, Vroom TM, et al. Extensive islet amyloid formation is induced by development of Type II diabetes mellitus and contributes to its progression: pathogenesis of diabetes in a mouse model. Diabetologia 1999;42:427-34. [92] Butler AE, Janson J, Soeller WC, Butler PC. Increased β-cell apoptosis prevents adaptive increase in β-cell mass in mouse model of type 2 diabetes: evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes 2003;52:2304-14. [93] Wong WPS, Scott DW, Ferreira A, Chuang CL, Zhang S, Liu H, et al. Spontaneous diabetes in hemizygous human amylin transgenic mice that developed neither islet amyloid nor peripheral insulin resistance. Diabetes 2008;57:2737-44. [94] Cooper GJS, Day AJ, Willis AC, Roberts AN, Reid KBM, Leighton B. Amylin and the amylin gene: structure, function, and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta 1989;1014:247-58. [95] Westermark P, Engström U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci USA 1990;87:5036-40. [96] Howard CF, Jr. Longitudinal studies on the development of diabetes in individual Macaca nigra. Diabetologia 1986;29:301-6. [97] de Koning EJP, Bodkin NL, Hansen BC, Clark A. Diabetes mellitus in Macaca mulatta monkeys is characterised by islet amyloidosis and reduction in beta-cell population. Diabetologia 1993;36:378-84. [98] Ohagi S, Nishi M, Bell GI, Ensinck JW, Steiner DF. Sequences of islet amyloid polypeptide precursors of an Old World monkey, the pig-tailed macaque (Macaca nemestrina), and the dog (Canis familiaris). Diabetologia 1991;34:555-8. [99] Guardado-Mendoza R, Davalli AM, Chavez AO, Hubbard GB, Dick EJ, Majluf-Cruz A, et al. Pancreatic islet amyloidosis, β-cell apoptosis, and α-cell proliferation are determinants of islet remodeling in type-2 diabetic baboons. Proc Natl Acad Sci USA 2009;106:13992-7. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 37 [100] Green J, Goldsbury C, Mini T, Sunderji S, Frey P, Kistler J, et al. Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J Mol Biol 2003;326:1147-56. [101] Verchere CB, D’Alessio DA, Palmiter RD, Weir GC, Bonner-Weir S, Baskin DG, et al. Islet amyloid formation associated with hyperglycemia in transgenic mice with pancreatic β-cell expression of human islet amyloid polypeptide. Proc Natl Acad Sci USA 1996;93:3492-6. [102] Fox N, Schrementi J, Nishi M, Ohagi S, Chan SJ, Heisserman JA, et al. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM). FEBS Lett 1993;323:40-4. [103] Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature 1994;368:756-60. [104] Wang F, Hull RL, Vidal J, Cnop M, Kahn SE. Islet amyloid develops diffusely throughout the pancreas before becoming severe and replacing endocrine cells. Diabetes 2001;50:2514-20. [105] Haluzik M, Colombo C, Gavrilova O, Chua S, Wolf N, Chen M, et al. Genetic background (C57BL/6J versus FVB/N) strongly influences the severity of diabetes in insulin resistance in ob/ob mice. Endocrinol 2004;145:3258 -64. [106] Leighton B, Cooper GJS. Pancreatic amylin and calcitonin gene- related peptide cause resistance to insulin in skeletal muscle in vitro. Nature 1988;335:632-63. [107] Roberts AN, Leighton B, Todd JA, Cockburn D, Schofield PN, Sutton R, et al. Molecular and functional characterization of amylin, a peptide associated with type 2 diabetes mellitus. Proc Natl Acad Sci USA 1989;86:9662-6. [108] Cooper GJS. Amylin: physiology and pathophysiology. In: Jefferson LS, Cherrington AD, editors. The Handbook of Physiology, Section 7 The Endocrine Pancreas and Regulation of Metabolism. New York, N. Y.: American Physiological Society, Oxford University Press, 2001. p. 303-96. [109] Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes 1999;48:491-8. [110] Porat Y, Kolusheva S, Jelinek R, Gazit E. The human islet amyloid polypeptide forms transient membrane-active prefibrillar assemblies. Biochem Biophys Res Commun 2003;42:10971-7. [111] Petyuk VA, Qian WJ, Hinault C, Gritsenko MA, Singhal M, Monroe ME, et al. Characterization of the mouse pancreatic islet proteome and comparative analysis with other mouse tissues. J Proteome Res 2008;7:3114-26. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 38 [112] Metz TO, Jacobs JM, Gritsenko MA, Fontès G, Qian WJ, Camp DG, et al. Characterization of the human pancreatic islet proteome by two-dimensional LC/MS/MS. J Proteome Res 2006;5:3345-54. [113] Johnson JD, Bernal-Mizrachi E, Alejandro EU, Han Z, Kalynyak TB, Li H, et al. Insulin protects islets from apoptosis via Pdx1 and specific changes in the human islet proteome. Proc Natl Acad Sci USA 2006;103 19575-80. [114] Buchanan CM, Phillips AR, Cooper GJS. Preptin derived from proinsulin-like growth factor II (proIGF-II) is secreted from pancreatic islet beta-cells and enhances insulin secretion. Biochem J 2001;360:431-9. [115] Durrieu C, Bernier-Valentin F, Rousset B. Binding of glyceraldehyde 3-phosphate dehydrogenase to microtubules. Mol Cell Biochem 1987;74:55-65. [116] Rojiani M, Finlay B, Gray V, Dedhar S. In vitro interaction of a polypeptide homologous to human Ro/SS-A autoantigen (calreticulin) with a highly conserved amino acid sequence in the cytoplasmic domain of integrin alpha subunit. Biochemistry 1991;30:9859-65. [117] Bleackley R, Atkinson E, Burns K, Michalak M. Calreticulin: a granule-protein by default or design. Curr Top Microbiol Immunol 1995;198:145-59. [118] Baksh S, Michalak M. Expression of calreticulin in Escherichia coli and identification of its Ca2+ binding domains. J Biol Chem 1991;66:21458-65. [119] Nakamura M, Moriya M, Baba T, Michikawa Y, Yamanobe T, Arai K, et al. An endoplasmic reticulum protein, calreticulin, is transported into the acrosome of rat sperm. Exp Cell Res 1993;205:101-10. [120] Soeyoshi T, McMullen B, Marnell L, Clos T, Kisiel W. A new procedure for separation of protein Z, prothrombin fragment 1.2 and calreticulin from human plasma. Thromb Res 1991;63:569-75. [121] Orci L. The insulin cell: its cellular environment and how it processes (pro)insulin. Diabetes Metab Rev 1986;2 (1-2):71-106. [122] Hutton JC, Penn E, Peshavaria M. Low-molecular-weight constituents of isolated insulinsecretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J 1983;210:297-305. [123] Foster LJ, de Hoog CL, Zhang Y, Xie XM, Vamsi K, Matthias M. A mammalian organelle map by protein correlation profiling. Cell 2006;125:187-99. [124] Martinez LO, Jacquet S, Esteve JP, Rolland C, Cabezon E, Champagne E, et al. Ectopic beta-chain of ATP synthase proteins is an apolipoprotein A-I receptor in hepatic HDL endocytosis. Nature 2003;421:75-9. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 39 [125] Freddo TF. Mitochondria attached to desmosomes in the ciliary epithelia of human, monkey, and rabbit eyes. Cell Tissue Res 1988;251:671-5. [126] Rudolf R, Salm T, Rustom A, Gerdes HH. Dynamics of immature secretory granules: role of cytoskeletal elements during transport, cortical restriction, and F-Actin-dependent tethering. Mol Biol Cell 2001;12:1353-65. [127] Mootha VK, Bunkenborg J, Olsen JV, Hjerrild M, Wisniewski JR, Stahl E, et al. Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 2003;115:629-40. [128] Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev 2003;83:581-632. [129] Maechler P, Wollheim C. Mitochondrial function in normal and diabetic β-cells. Nature 2001;414:807-12. [130] Ahmed M, Bergsten P. Glucose-induced changes of multiple mouse islet proteins analysed by two-dimensional gel electrophoresis and mass spectrometry. Diabetologia 2005;48:477-85. [131] Arias AE, Vélez-Granell CS, Mayer G, Bendayan M. Colocalization of chaperone Cpn60, proinsulin and convertase PC1 within immature secretory granules of insulinsecreting cells suggests a role for Cpn60 in insulin processing. J Cell Sci 2000;113:207583. [132] Brudzynski K, Martinez V. Synaptophysin-containing microvesicles transport heat-shock protein hsp60 in insulin-secreting beta cells. Cytotechnol 1993;11:23-33. [133] Tytell M. Release of heat shock proteins (Hsps) and the effects of extracellular Hsps on neural cells and tissues. Int J Hyperthermia 2005;21:445-55. [134] Macario AJL, Conway de Macario EC. Mechanisms of disease: sick chaperones, cellular stress and disease. N Engl J Med 2005;353:1489-501. [135] Lad RP, Smith MA, Hilt DC. Molecular cloning and regional distribution of rat brain cyclophilin. Mol Brain Res 1991;9:239-44. [136] Tribl F, Gerlach M, Marcus K, Asan E, Tatschner T, Arzberger T, et al. “Subcellular Proteomics” of neuro-melanin granules isolated from the human brain. Mol Cell Proteomics 2005;4:945-57. [137] Nayeen MS, Khan RH. Misfolded proteins and human diseases. Protein Pept Lett 2004;11:593-600. [138] Paulsson JF, Westermark GT. Aberrant processing of human proislet amyloid polypeptide results in increased amyloid formation. Diabetes 2005;54:2117-25. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 40 [139] Saafi E, Konarkowska B, Zhang S, Kistler J, Cooper GJS. Ultrastructural evidence that apoptosis is the mechanism by which human amylin evokes death in RINm5F pancreatic islet β-cells. Cell Biol Int 2001;25:339-50. [140] Ross SA, Gulve EA, Wang M. Chemistry and biochemistry of type 2 diabetes. Chem Rev 2004;104:1255-82. [141] Aitken JF, Loomes KM, Konarkowska B, Cooper GJS. Suppression of the conversion of human amylin into insoluble amyloid by polycyclic compounds. Biochem J 2003;374:779-84. Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 41 7. Figure Legends Figure 1 Microscopic view of an original haematoxylin and eosin-stained pancreatic section wherein islet ‘hyaline’ (now known as amyloid) was first identified by Dr Eugene Opie, in a post-mortem study of a patient with type-2 diabetes mellitus [23]. The islets, which are usually replete with endocrine cells, have largely been replaced by the amorphous, faintly pink-staining islet amyloid. [Reproduced with permission of Robert D. Hoffman, M.D., Ph.D., Department of Biological Chemistry, The Johns Hopkins University School of Medicine, Baltimore, MD]. Figure 2 Electron micrographs illustrating secretory granules from cultured murine insulinsecreting βC6-F7 cells. A, Structures in the perinuclear region, show characteristic membranelimited granules in different stages of maturation (arrowed). B, Mature secretory granules near the cellular periphery show characteristic electron-dense cores and adjacent electron-lucent haloes (arrowed). Abbreviation: N, nucleus; Bars = 300 nm. (Reproduced with permission from C. M. Buchanan et al.: Biochem J 360, 431, 2001 [114]). Figure 3 Original chromatographic purification of human amylin from pancreatic extracts of type-2 diabetic patients provides an early example of the application of comparative proteomic analysis to tissues. A, HPLC gel filtration in 6 M guanidine hydrochloride of an extract from an amyloid-containing pancreas taken at post mortem from a patient with type-2 diabetes. Amylin was present in the region indicated by the bar. B, Reversed-phase HPLC of material from the region indicated by the bar in A: unreduced amylin was present in peak 3. C, Reversed-phase Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 42 HPLC of a control extract from a control pancreas from a nondiabetic patient, as in B. Peaks 1 and 2 corresponded in elution time and amino acid composition to 1 and 2 in B. D, Repurification by reversed-phase chromatography of peak 3B after reduction and alkylation of cysteine residues and [14C]-radiolabelling of cysteinyl residues. Peaks 4 and 5 had amino acid compositions distinct from that of 3RA, which was reduced and alkylated amylin. E, Separation of product peptides after tryptic digestion of reduced and alkylated amylin by reversed-phase HPLC. Peak 6 was the smaller, more hydrophilic peptide amylin1-11, and peak 7 the larger, more hydrophobic amylin12-37. All radiolabel was present in peak 6. Identity of peaks was confirmed by quantitative amino acid analysis and by gas-phase peptide sequencing. The ratio of peak heights is consistent with the relative lengths of the amylin-derived peptides. (Reproduced with permission from G. J. S. Cooper et al.: Proc Natl Acad Sci USA 84, 8628, 1987 [27]). Figure 4 Time-lapse atomic force microscopy (AFM) showing a human amylin oligomer growing into a fibril. Droplets of a human amylin solution were placed on a mica surface and studied by AFM using published methods [31]. Oligomers are seen to grow in height prior to extensive elongation into fibrils, and consist of ~16 monomers when first visualized (left-hand panel). The height of the oligomer (arrow) is seen to increase with each scan. The time points and height (h) and length (l) measurements are as indicated in each image. (Reproduced with permission from J. D. Green et al.: J Biol Chem 279, 12206, 2004 [31]). Figure 5 Amyloid visualized by light microscopy was dissociable from occurrence of diabetes in hemizygous human amylin transgenic mice. Photomicrographs show serial pancreatic islet Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 43 sections from non-transgenic and human amylin transgenic animals. Left photomicrographs from top three panels show insulin- (green) and glucagon- (red) immunoreactivity. Bottom two left panels show islet sections incubated with antisera to somatostatin and glucagon, revealing brown cytoplasmic staining. Middle and right panels show corresponding light- and polarizedmicroscopic field views of adjacent islet sections stained with Congo red. Amyloid birefringence is apple green whereas that corresponding to collagen is silvery. The scale bar (50 μm) shown in top left photomicrograph applies to all images except for those corresponding to the 600 day non-diabetic hemizygous mouse (second to bottom row) which represents 100 μm. (Reproduced with permission from J. F. Aitken et al.: Diabetes, Epub ahead of print September 30th, 2009; doi:10.2337/db09-0548 [32]). Figure 6 Human amylin extracted from the pancreas of type-2 diabetic patients elicits dosedependent inhibition of insulin-stimulated glycogen synthesis in ex vivo rat soleus muscle. The human amylin used in these studies was purified and characterised by the methods of Cooper et al. [27]. Values shown are means of at least four separate incubations. Statistically-significant (p < 0.05, Student’s t-test) decreases from control values are indicated by *, (control against 10-9 M amylin); †, (10-9 M amylin against 10-8 M amylin). Methods. Muscle preparation and incubation methods were as described [106]. Typically, a submaximal concentration of insulin (100 μU.ml-1) stimulates the rate of glycolysis and glycogen synthesis, by ~50% and 125% above basal rates respectively, in this preparation. (Reproduced with permission from B. Leighton and G. J. S. Cooper: Nature 335, 632, 1988 [106]). Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 44 Figure 7 Fractionation of lysates from cultured INS-1E β-cells was monitored by serial enzyme or hormone assays, as described [20]. Markers used were NADH cytochrome C reductase (endoplasmic reticulum), citrate synthase (mitochondria), lactate dehydrogenase (cytosol), insulin (granules), and aryl sulphatase (lysosomes). Insulin was concentrated within specific fractions (broken line). Insulin-containing fractions were then subjected to immunoaffinity purification using VAMP2. (Reproduced with permission from A. R. Hickey et al.: J Proteome Res 8, 178, 2009 [20]). Abbreviations: VAMP, vesicle-associated membrane protein Figure 8 Pie chart summarizing the cellular location of proteins identified in anti-VAMP2 antibody-conjugated magnetic Dynal bead-purified granule proteins, as listed in Table 2. (Reproduced with permission from A. R. Hickey et al.: J Proteome Res 8, 178, 2009 [20]). Abbreviations: VAMP, vesicle-associated membrane protein Garth J S Cooper, School of Biological Sciences, University of Auckland, Private Bag 92-019, Auckland, New Zealand E-mail: g.cooper@auckland.ac.nz 45