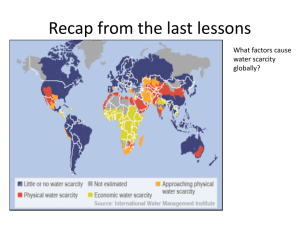
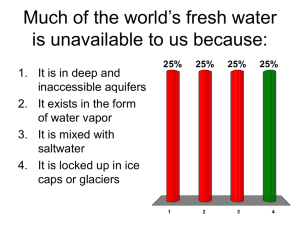
Final Report
advertisement

1. INTRODUCTION (1-2 pages) This chapter contains some background information on the specific situation in the country that led to proposing this SfP Project. Special attention should be given to the economic and industrial benefits that are expected as a result of this Project as stated in the Criteria for Success Table, including its contribution to strengthening the national scientific/technological infrastructure. This information should be quantified, where possible. The ecological devastation and economic impoverishment that have befallen the Aral Sea communities are threats to the security of the region and to the health and well-being of the people. There is an immediate need for the communities surrounding the Aral Sea to improve their quality of life through economic opportunities. The Aral Sea ecological change has resulted in total obliteration of the fish stocks upon which these communities formerly depended for their sustenance and employment, and the contemporary East and West parts of the so-called ‘Big’ Aral are essentially hypersaline water bodies with near-total elimination of species of fresh, brackishwater, or marine origin. The likelihood of recovery of fish stocks to these parts of the Aral Sea is very low. Water management alternatives, the introduction of drought tolerant crop strains, less wasteful irrigation methods and other means of mitigating the economic losses and ecological demise of the region may hold some promise for the Aral Sea, but they have been largely unsuccessful. Water management construction works in the area of the so-called ‘Small’ Aral (in the north; Kazakh territory) have resulted in a stabilization or even partial restoration of former salinity levels, but have virtually no impact on the Big Aral. Similar, but even bigger-scaled hydrological engineering works are needed for the stabilization or partial restoration of the Big Aral, but both funding and political consensus are not available within the foreseeable future. Any means of economic recovery for the Aral Basin communities would therefore be a most welcome and much needed benefit. There is evidence of a recently colonizing population of the halophilic Artemia in the Aral Sea that holds promise as a commercially viable resource. The development and prudent management of an Aral Sea Artemia resource could provide some degree of economic and ecological recovery to the region through sustainable management strategies. Artemia is an essential component of aquaculture production worldwide. Global demand for Artemia biomass and cysts is on the order of approximately 2000 tonnes per year. Revenues from the sale of Artemia products are in the range of 55 to 95 million USD per year. Over the course of the past two decades the demand for aquaculture products has increased at a rate of 5% to 10% per year. The potential economic benefits gained through successful development of the emerging Aral Sea Artemia resource could provide the people of the region with employment and other opportunities that presently do not exist, contributing to local social security. Additionally, secondary benefits arising out of demands for goods and services and an improved infrastructure may follow. However, it is insufficiently known whether the current hydrobiological and hydrochemical status and primary productivity of the Aral Sea is sufficient to support a stable Artemia population. It is therefore of crucial importance to implement an ecological monitoring program to determine the potential suitability of the Aral Sea as an environment in which one could expect sustained Artemia population growth and productivity. Furthermore, the potential for commercial exploitation will depend on Artemia population dynamics: a consistent high annual rate of cyst production, coupled with high cyst quality, and a low cost of production could certainly result in a marketable export product for the region. Expert advice in harvesting, transport, handling, storage, and industrial production techniques are additionally essential for the successful exploitation of the Artemia resource. The research team for this project includes some of the world’s foremost experts in Artemia biology and ecology. State-of-the-art methods for characterizing Artemia, conducting population studies, water quality and phytoplankton assessments in use by scientists from NATO countries will be introduced to partner country scientists. Well-established sustainable management practices developed by the NATO project partners for Great Salt Lake Artemia, Utah, USA and successfully applied for Artemia populations in Russia, Turkmenistan, Kazakhstan and China, will serve as a model for an Aral Sea Artemia management strategy. Monitoring devices for sampling and water 1 quality determination, Artemia cyst harvesting, storage, processing and quality control, provide significant advancements in research efficiency for partner country scientists. Population models of the Great Salt Lake (GSL) Artemia have been developed and illustrate the economic and conservation benefits of thorough research and well-formulated interpretation of the scientific data. Population growth models of the GSL Artemia resource have proven to be reasonably accurate predictors of harvest quantities over the past six years. Harvestable quantities are an important aspect to evaluate the exploitation potential of a site, but quality and application range of the marketable product also decisively determines the potential yield. The average quality of cysts harvested from diverse geographical regions of the world varies considerably. It is a function of the inherent quality of the cysts and, equally important, handling, storage, and processing methods. At one extreme are the cysts from the GSL; skilled harvesters and processors can produce dried cysts with an overall average quality of over 85% for all harvested cysts. Cysts from other locations may have a maximum quality of 70% hatching percentage with the majority of harvested cysts exhibiting a range of lower qualities. Quantities from other sources (China, Russia, Central Asia) have shown improved consistency in quality and yields over the past 5 years and demand for alternative Artemia cysts will is directly related to the production at GSL. It is unknown whether cysts from the Aral Sea will demonstrate high average quality. The market for Central Asian cysts is generally well below that for alternative sources of cysts, resulting in a decrease in the expected value. Buyers’ perception of quality plays a key role in the value of Artemia cysts. Newly introduced cyst strains are often valued less than known sources, even with comparable quality characteristics. It is also for this reason that exploitation and marketing of Aral Sea Artemia cysts should be supported by the best science available. 2 2. SCOPE AND OBJECTIVES OF THE PROJECT (1-3 pages) This chapter contains a summary of the objectives to be achieved within the lifetime of the Project. - scientific goals - participation by other national institutions/industries - training - enhancement of scientific infrastructure - international co-operation - etc. Scientific goals 1. Description and characterization of the Aral Sea Artemia population: biology (incl. life history studies), population dynamics, cyst quality characteristics and their potential for aquaculture 1.1. Field research hydrobiological and hydrochemical characterization of the Aral Sea: salinity; ion cmposition, temperature phytoplankton density and species composition documenting and modeling Artemia population dynamics: age class composition; fecundity, other zooplankton species: presence/absence studies of predatory or competitor zooplankton 1.2. Laboratory experiments phytoplankton: isolation, identification and growth of Aral Sea algal species at different abiotic conditions: light, temperature, nutrient levels Artemia: o strain characteristics and quality control: cyst biometrics, hatching quality, diapause behaviour, nutritional characteristics (HUFA profile) o life history and reproductive characteristics; effect of abiotic conditions temperature, salinity, feeding o predation on Artemia nauplii by other zooplankton organisms 2. Development of a population model for Aral Artemia resource. Population models of the Great Salt Lake (GSL) Artemia have been developed and have proven to be reasonably accurate predictors of harvest quantities. A similar model should be developed for the Aral Sea Artemia, that can be used for resource managers and for determining the commercial viability. 3. Resource management recommendations: define sustainable management plan; sustainable is defined in terms of consistently maintaining a commercial operation and striving to ensure that harvesting efforts do not result in adverse impacts on cyst production. Determine commercial viability and outline optimal means of exploitation of the Artemia resource. Through documentation of Artemia population dynamics, Aral Sea hydrology and hydrochemistry, and micro-algae analyses, potential production of cysts can be estimated. Therefore experimental harvests can be conducted to provide information on: correlation between standing crop estimates and shoreline quantities available for harvesting; harvesting and processing yields; logistical consideration, especially transport time and constraints; cyst quality and optimal means of handling, storage, and processing. Above information will be used in conjunction with Artemia market information to describe the economic viability of the resource, and to issue recommendations towards end-users on optimal utilisation of resource: site-specific strategies for harvest, transport, and processing, potential economic benefit, estimation of employment generated by sustainable Artemia cyst industry, and cost/benefit analysis. 3 Participation by other national institutions/industries The project members envisage the practical use of the project’s realizations by the implementation of the project’s results by the end-users. The latter consist of national, regional or local decision-making authorities, and of institutes and other governnmental entities involved in activities related to some aspect of the study and exploitation of the broad Aral Sea socio-economic and biological environment (e.g. local employment, fisheries, water management, environmental issues…). These end-users have expressed their interest through a Letter of Intent at the beginning of the project, and are involved in the progress of the project through the organization of workshops in Tashkent by the project partners. During these workshops, the end-users are informed about the project’s findings, and are invited to provide feedback aiming at adjustment and optimization of the project’s activities. During the Concluding Workshop, organized in the final phase of the project, they will be informed about the overall results of the project. Training Transfer of know-how is an integral and essential part of this project; training of partner institutes in all techniques needed to attain objectives 1-2-3, detailed above, and to realize a sustainable economic exploitation of Aral Sea Artemia, will be organized. This implies: Training of Uzbek partners at NATO partner’s facilities o hydrochemistry: methods of hydrochemical water analysis, with focus on nutrients analysis in saline environment o phytoplankton: methods of sampling, preservation and processing of phytoplanktonic and microphytobenthic organisms; isolation, establishing and maintenance of monoclonal cultures of microalgae; principles and methods of modern taxonomic identification of aquatic microorganisms o Artemia: biology, use in aquaculture, morphology and life cycle, ecology, cyst quality control diapause termination, decapsulation, enrichment; culture tests to study life cycle and reproductive characteristics at different abiotic and food conditions; commercial harvesting, storage and processing of Artemia cysts; interpretation of population dynamics data; population modeling. Training of Uzbek partners in the field at Aral Sea: hydrochemical and hydrobiological sampling, quantitative and qualitative sample analysis, data processing and interpretation Enhancement of scientific infrastructure The project aims at the enhancement of scientific infrastructure of the Uzbek partner institutes by purchase, transport and installation of scientific equipment Laboratory equipment: all equipment (and consumables) needed to conduct the laborary work described above; training will be provided in the operation and use of these equipment items; Field equipment: all equipment, infrastructure and vehicles (and consumables) needed to conduct the field study (hydrochemistry, primary production, Artemia, zooplankton) described above; i.e. material needed for sample-taking from the Aral Sea, field sample analysis and transport of the samples to the laboratory. International cooperation Although international cooperation beyond the project partners is not a direct objective of the project, the Uzbek partners will benefit from an increased visibility on the international forum (which may provide leverage for faciliting international cooperation in the future) through their intense contacts with the NATO country partners, which are thoroughly embedded in the international scientific forum. 4 5 3. REALIZATION OF THE PROJECT (1-3 pages) An overview of the organisation of the Project, the management structure, and the participating institutions and industries. If the Project is composed of several sub-projects, these items should be treated for each sub-project. Participating institutions NATO countries: Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Rozier 44, B9000 Gent, Belgium (ARC) (NPD: Prof. P. Sorgeloos; co-director Dr. G. Van Stappen) Laboratory of Protistology & Aquatic Ecology, Department of Biology, Ghent University, Krijgslaan, 281-58, B9000 Gent, Belgium (LPAE) (co-director Prof. W. Vyverman) Utah Strategic Alliance (UtSA) (renamed into Great Salt Lake Artemia Cooperative in 2006), 5859; N. Cottonwood Canyon Rd. Mt. Green, UT, USA (co-director Th. Bosteels) INVE Technologies (INVE Tech), Artemia Task Force, PO Box 1306, 598 W Clark St. Grantsville, UT, USA (co-director B. Marden) Uzbekistan: Laboratory of Ichthyology and Hydrobiology, Institute of Zoology, Uzbek Academy of Sciences, Niyazov st. 1, Tashkent, 700095 (LIH) (PPD: Prof. I. Mirabdullayev) Institute of Bioecology of the Karakalpak Branch of the Uzbek Academy of Sciences; 41, Berdakh prospect, 742000, Nukus (IB) (co-director Dr. I. Zholdasova) Management structure see below project organigram 6 Organisation of project List of (categories of) persons involved in project, their affiliation, and a summary of their tasks and responsibiilties Identity Patrick (NPD) Affiliation Tasks in the project Sorgeloos ARC Iskandar Mirabdullayev (PPD) LIH Co-directors NATO countries (Brad Marden, Gilbert Van Stappen, Thomas Bosteels, Wim Vyverman) Co-directors partner country (Iliya Zholdasova) INVE Tech, ARC, UtSA, LPAE (resp.) IB Other scientific personnel (esp. Uzbek young scientists) LIH, IB Logistic support personnel INVE External NATO advisors S. Bradt1, D. Naftz², R. Baskin² End-users 1 Univ. New Hampshire, USA; ²US Geological Survey general project coordination feedback to milestones, deliverables, reports coordination of NATO country project partners co-organizer of workshops end-user feedback to milestones, deliverables, reports organizer of workshops coordination of partner country project partners: field and lab work; organization of training missions to NATO countries daily management of project, partim LIH daily management of project organization of training of Uzbek scientists at NATO partners institutes field training of Uzbek scientists in Uzbekistan co-reporting participation to workshops daily management of project co-reporting participation to workshops interaction of project consortium with regional (Karakalpakistan) and local (Moinaq) level conduct laboratory tests, field sampling and sample analysis participation to workshops contribution to reporting through compilation of field and laboratory work reports target of training assistance in field sampling translation of documents and interpreter during workshops participation to workshops advise in specific scientific issues feedback on reports participation to workshops authorities at different provide feedback to project results levels participation to workshops institutes/organisations where relevant, facilitate and promote project involved in Aral Sea activities management & study 1 4..SCIENTIFIC RESULTS (up to 40 pages) The scientific results are reported here in the nature of an article in a scientific periodical or as a presentation at a scientific conference. As a consequence, this reporting is relatively detailed and may be organised per sub-project. Special attention should be given to the need for further R&D activities after conclusion of the Project. PART 1. FIELD STUDIES Asf Jedeli Vozrojdeniya JA W Akt E E a s t Lazarevo Moinak Artemia of the Aral Sea, Uzbekistan. G. Van Stappen et al. 10th Int. Conf. on Salt Lake Research. Salt Lake City, USA, May 11-16, 2008 slide 17 of 28 1. HYDROCHEMICAL COMPOSITION OF THE ARAL SEA IN THE PERIOD 2005-2008 2 30 temperature (°C) 25 20 15 10 5 West Aral 0 Jan-04 Aug-04 Feb-05 Sep-05 Mar-06 East Aral Oct-06 Apr-07 Nov-07 month-year 1.1. Ionic composition of East and West Aral surface waters In the first half of the twentieth century, the salinity of water in the open part of the Aral Sea reached about 10 ppt. In the period 1960-1980, the salinity increased at an average rate of 0.2-0.6 ppt per year (Table 1.1). Since 1989, the Aral Sea split into the Large and Small Aral and the salinity started growing at the rate of 1 ppt per year. In the 1990’s, the central range of islands (Vozrozhdeniye and Lazarev Islands) merged into one island, which caused a separation of the Big Aral into the western and eastern basins. In recent years, the salinity of the water in the western basin increased at an average rate of 5-10 ppt per year, while in the shallow eastern basin the rate of salinity increase 5-20 ppt per year (Table 1.1). In 2005-2007 the salinity of the West Aral (which is relatively deep) increased relatively fluently reaching 116 ppt at the end of 2007 (Fig. 1.1). At the same time the salinity in the shallow East Aral underwent sharp fluctuations, caused by fluctuations of inflow from the Amu Darya delta. So, salinity in the East Aral reached 150 ppt in 2002, and then dropped to less then 100 ppt in 2003. In August 2005 a lot of water came into the East Aral causing a decreasing salinity till 67 ppt. In the relatively dry period 2006-2007 salinity quickly increased again, reaching 265 ppt in coastal areas of the East Aral in May 2007 causing crystallization of sulphates (Fig. 1.2). Apart from this, nu substantial changes in ion composition were observed over the period 2005-2007, nor between the East and West Aral, as the precipitation point for salts had not been reached. Table 1.1. Salinity in Aral Sea surface water in period 1960-2007 Year 1960 1970 1975 1980 1985 1990 1992 1995 1996 Salinity (ppt) 10 12 14 17 23 32 35 42 44 West Aral 3 East Aral 1997 49–51 50–52 1998 54 58 1999 56 2000 58-63 2001 63-68 108-112 2002 69-74 150 2003 74-80 90-110 2004 84-94 90-116 2005 82-92 67-113 2006 96-101 70-136 2007 95-116 (265)* *As the East Aral was virtually inaccessible for regular sampling taking in 2007, this value corresponds with highly saline coastal samples 140 120 salinity (g/l) 100 80 60 40 West Aral East Aral 20 0 Jan-04 Aug-04 Feb-05 Sep-05 Mar-06 Oct-06 month-year Fig.1.1. Salinity fluctuations in Aral Sea surface water in period 2004-2007 4 Apr-07 Nov-07 Dynamic of ion composition of water in East Aral 100% 80% 60% 40% 20% 2005 2006 HCO3 Cl SO4 Aral Ca++ East Sea Mg++ June 2007 Na+K Fig. 2.1. Dynamics of East Aral surface water ion composition in period 2005-2007 5 September August, 2 part August, 1 part June May April November October September August 0% Dynamic of ion composition of water in West Aral 100% 80% 60% 40% 20% 2005 2006 October- September August July June, 2 June, 1 May September August-2 August-1 June May April November October September August July 0% 2007 West Aral Sea HCO3 Cl SO4 Ca++ Mg++ Na+K Fig. 1.3. Dynamics of West Aral surface water ion composition in period 2005-2007 1.2. Nutrient composition of deep West Aral water layers In view of the low overall phytoplankton concentrations observed in West and East Aral (see 2.) nutrient analysis (N and P) was performed for water samples taken from various depths in the West Aral, in order to verify if a chemocline existed for these compounds. In the positive case, the project partners might consider the possibility of valorising nutrient-rich deep-layer water using air-water lifts for increased primary (and hence secondary) production in superficial water layers. Samples were taken on 3 sites (Aktumsuk- AKT, Jidely Bulak-JID, Asphalt Kulau-ASP) at the western shore, at 3 and 4 km offshore (S1 and S2, resp.), at different depths: 1 m, 10 m, and nearbottom. As shown in Table 1.2., N, P and Fe levels were largely homogeneous throughout the water column, or if variations occurred, these were not consistent with depth. 6 Table 1.2. Nutrient levels in deep-water samples taken along West Aral western coast Sampling Site AKT S1 Total N (mg/l)1 2.18 2.80 2.43 2.34 2.46 2.52 2.72 2.98 2.59 2.51 2.57 2.82 2.87 2.77 2.65 2.80 2.92 3.04 Depth (m) 1 10 23 S2 1 10 25 JID S1 1 10 33 S2 1 10 37 ASP S1 1 10 36 S2 1 10 32 1 detection limit 0.050 mg/l ²detection limit 0.002 mg/l ³detection limit 0.020 mg/l Total P (mg/l)² 0.048 0.277 0.078 0.039 0.041 0.077 0.046 0.068 0.055 0.041 0.041 0.045 0.042 0.040 0.041 0.038 0.039 0.115 Iron (mg/l)³ 0.046 0.298 0.261 <0.020 <0.020 0.060 0.030 0.110 0.057 <0.020 <0.020 0.028 <0.020 <0.020 <0.020 <0.020 0.050 0.065 2. EVOLUTION OF THE PHYTOPLANKTON DENSITY AND SPECIES COMPOSITION IN THE ARAL SEA IN THE PERIOD 2005-2007 2.1. Introduction Phytoplankton of the Aral Sea was studied since the beginning of the 20th century (Kiselev, 1927). Later on data on phytoplankton were presented by Pichkily (1981), Оrlova et al. (1998) and Mirabdullayev et al. (2004). During the progressive salinization of the Aral Sea, the diversity of planktonic algae has continuously decreased, being in the last years about 4 times lower than 80 years ago (Table 2.1). Table 2.1. Number of phytoplankton species found in the Aral Sea during its transition from a oligohaline to a hypersaline environment Тахon Сyanobacteria 41 1967 – 1974² 79 Bacillariophyta 210 104 115 41 49 56 Pyrrophyta 15 28 3 3 4 3 Euglenophyta 0 3 2 0 0 2 19251 1999-2002³ 2005 2006 2007 30 14 15 15 7 Chlorophyta 109 60 9 12 11 14 Total number 375 306 159 70 of species 1 Kiselev (1927); ²Pichkily (1981), ³Mirabdullayev et al. (2004) 79 90 Due to the high water transparency and shallow depths in the Aral Sea, most organics have been produced by phytobenthos, not phytoplankton. In general, the biomass of phytobenthos reached 90%, while phytoplankton reached 10% (Karpevich, 1975). Charophytes (mainly) yielded ca. 75% and the chlorophyte ca. 13% of the phytobenthos biomass. In the 1990’s common phytobenthos such as Tolypella aralica, Vauscheria dichotoma, Cladophora gracilis, Polysiphonia violaceae and Zostera (Karpevich, 1975) became extinct in the Aral Sea. Presently the only benthic macroscopic plants in the Aral are Cladophora glomerata, C. fraсta and Vauscheria cf. dichotoma (Zavialov et al., 2006). 2.2. Material and methods Phytoplankton samples were fixed by lugol, and postfixed a few minutes later with formaldehyde at 0.5%. Samples were filtered using Millipore filters (1,2 μm) and counted in a Najot chamber under a Zeiss Axiostar microscope. In 2005-2006 samples were collected along the western shore of the Eastern Aral (depths 1.0-2.5 m) and the eastern shore of the West Aral (depths 1.0-5.0 m). In 2007 samples were only collected on the western shore of the West Aral (depths 1.0-40.0 m). A total of 132 samples were collected and analysed. 2.3. Results During the project lifetime 90 species of algae were recorded in total in the Aral Sea plankton (Table 2.2). Most species recorded belong to the diatoms (Bacillariophyta) which constituted 57-64% of all species (Fig. 2.1). Also important were such groups as Cyanobacteria (17-22%) and Chlorophyta (1318%). Other algal phyla were represented by single species. The species composition of phytoplankton in West and East Aral was largely similar. Not all recorded algal species are truly planktonic. As the collection sites were shallow (2-4 m), a significant number of algal species are representatives of phytobenthos and periphyton. Table 2.2. Phytoplankton species observed in West and East Aral in period 2005-2007 Taxon CYANOBACTERIA 1.Microcystis aeruginosa Kutz 2.Oscillatoria sp.(limosa?) 3.O.. chlorina(Kutz) Gom. 4.O.. planctonica Wolocz. 5.O.. amphibia Ag. 6.Merismopedia glauca (Ehr.)Nag. 7.Gloeocapsa alpina Nag.em.Band 8. G. alpina f. lignicola (Rabenh.) 9.G. minima (Keissl.) 10. G. minor (Kutz) 11.G. turgida (Kutz.) 12.Gomphasphaeria aponina Kutz. 13.G.. lacustris Chod. 14.Synechococcus sp.(salina?) 2005 2006 2007 East Aral West Aral East Aral West Aral West Aral + + + + + + + + + + + + + - 8 + + + + + + + + + + + + + + + + + + + + + + + 15.Phormidium ambiguum Kissel 16.Phormidium sp. 17.Ph. papillaterminatum Kissel. 18.Lyngbya sp. 19.L. Kuetzingii (Kutz.) Schmidle 20.Spirulina sp. 21.Spirulina major Kutz. 22.Anabaena flos-aquae (Spreng) BACILLARIOPHYTA 1.Cyclotella sp. 2.C. meneghiniana Kutz. 3.Melosira varians Ag. 4.Rhizosolea longiseta Zacharias 5.Synedra tabulata (Ag.) Kutz. 6.S. tabulata v. parva (Kutz) Grun. 7.S. tabulata v. fasciculata (Kutz.) 8.Synedra sp. 9.S. minuscula Grun. 10.Fragilaria construens (Ehr.) Grun 11.F. .construens v. venter (Ehr.) Grun. 12.Actinocyclops Ehrebergii 13.Achnanthes minutissima Kutz. 14.A. affinis Grun. 15.Achnanthes sp. 16.Chaetoceros sp. 17.Coscinodiscus sp. 18.Stephanodiscus sp. 19.Cocconeis sp. 20.Cocconeis placentula Ehr. 21.C. placentula v.euglypta (Ehr.) Cl. 22.Diploneis intterupta (Kutz.) 23.D. Smithii (Breb) Cl. 24.D. Smithii v .pumila (Grun.) Hust. 25.Entomoneis paludosa Reimer 26.Amphora holsatica Hust. 27.A. coffeaformis Ag. 28.A. commutata Grun. 29.A. ovalis Kutz. 30.A.veneta Kutz. 31.A .robusta Greg. 32.Amphora sp. (long) 33.Mastogloia baltica Grun. 34.M. Smithii Thw. 35.M. pumilla (Grun.) Cl. 36.Navicula cincta (Ehr.) Kutz. 37.Navicula sp. 38.N. cryptocephala Kutz. 39.N. cryptocephala v.veneta Kutz. 40.N. kolbei Poretz et Aniss 41.N. protracta v.capitata Woronich. 42.N. pygmae Kutz. 43. N. salinarum Grun. + + + + - + + + + + + + + + + + + + + + + + + + + + + - + + + + + + + + + + + + + + + + + + + + + - + + + + + + + + + + + + + + + + + + + + + + + - + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + + 9 44. N. spicula Hickie 45. N. gracilis Ehr. 46. N. halophila (Grun.) Cl. 47. N. microcephala Grun. 48.Hantzshia virgata v.capitellata Hust. 49.Nitzschia closterium (Ehr)W.Sm. 50.N. acicularis W.Sm. 51.N. lorenziana v.incurta Grun. 52.N. microcephala Grun. 53.N. obtusa W.Sm. 54.N. obtusa v. scalpeliformis Grun. 55.N. cf .sigma (Kutz.) 56.N. palea (Kutz) W.Sm. 57.N. vermicularis (Kutz.) 58.N. punctata v.aralensis Borszczow 59.N. hungarica Grun. 60.N. capitellata Hust. 61.Gyrosigma scalproides (Rabenh) Cl. 62. G. acuminatum (Kutz) Raben 63.G. spenseri (W.Sm.) Cl. 64.Pleurosigma elongatum W.Sm. 65.P. obtuscurum W.Sm. 66.Surirella ovata Kutz. 67.Surilla sp.(.crumenosa?) 68.Campylodiscus aralensis Kissl.. PYRROPHYTA 1.Glenodinium sp. 2.Peridinium sp. CRYPTOHYTA 1.Cryptomonas sp. EUGLENOPHYTA 1.Euglena sp. 2.Phacus sp. CHLOROPHYTA 1.Chlorella sp. 2.Oocystis sp.(borgei Snow?) 3.O. marssonii Lemm. 4.Cosmarium formulosum Hoff. 5.Algae gen. sp. (Tetraselmis?) 6.Dunaliella sp. 7.Chlorocococcus turgida 8.Ankistrodesmus minutissima Korsch. 9.A. acicularis (Ag.Br.) 10.A. falcatus 11.Dactyosphaerium pulchellum Wood. 12.Tetraedron minimum (Ag.Br.) Hans. 13.Monas sp. 14.Carteria sp. 15.Chlorococcum sp. 16.Chlamidomonas sp. 17.Chromulina sp. 18.Polytoma sp.(ocellatum?) + + + + + + + + + + + + + + + + + + + - + + + + + + + + + + + + + + + + + + + + + + + + + + + - + + + + + + + + + + + + + + + + + + + + + + + - + - + + + - + + + - + + + - - - - + + + + + + + + + + + - + + + + + + - + + + + + + + + + - + + + + + + + + - + + + + + + + + + + + + + 10 19.Scenedesmus quadricauda 20.Cladophora fracta (L.) Kutz. Total number of species 56 + 47 West Aral 2005 70 + 54 + 69 East Aral 2005 West Aral 2006 East Aral 2006 West Aral 2007 Cyanobacteria Bacillariophyta Dinophyta Euglenophyta Cryptophyta Chlorophyta Fig. 2.1. Species diversity of phytoplankton in West and East Aral in period 2005-2007 Both the phytoplankton cell densities and biomass were normally higher in the West Aral than in the East Aral; the highest occurrence of cell density and biomass was observed in summer months (Figs 2.2 and 2.3). 11 2000 cell density per ml West Aral East Aral 1500 1000 500 Fe b -0 Ap 5 r-0 Ju 5 n0 Au 5 g0 O 5 ct D 05 ec -0 Fe 5 b0 Ap 6 r-0 Ju 6 n0 Au 6 g0 O 6 ct D 06 ec -0 Fe 6 b0 Ap 7 r-0 Ju 7 n0 Au 7 g0 O 7 ct -0 7 0 month-year Fig. 2.2. Seasonal and annual fluctuations in phytoplankton cell density in East and West Aral in the period 2005-2007 West Aral East Aral 140 biomass (mg/l) 120 100 80 60 40 20 Oct-07 Aug-07 Jun-07 Apr-07 Feb-07 Dec-06 Oct-06 Aug-06 Jun-06 Apr-06 Feb-06 Dec-05 Oct-05 Aug-05 Jun-05 Apr-05 Feb-05 0 month-year Fig. 2.3. Seasonal and annual fluctuations in phytoplankton biomass density in East and West Aral in the period 2005-2007 References Karpevich, A.F., 1975. Teoriya i praktika akklimatizatsii vodnikh organizmov. Pischevaya Promyshlennost, Moscow. 432 pp. (in Russian). 12 Kiselev I.A. 1927. Novye dannye o vodoroslyakh Aralskogo Morya. – Depart. Appl. Ichthyol. and Scient. Research. GIOA. 1927. V. 5. P. 1-64 (in Russian). Mirabdullayev I.M., Joldasova I.M., Mustafaeva Z.A., Kazakhbaev S., Lyubimova S.A., Tashmukhamedov B.A. 2004. Succession of the ecosystems of the Aral Sea during its transition from oligohaline to polyhaline waterbody. J. Marine Syst. V. 47. N 1-4. P. 101-107. Orlova M.I., Aladin N.V., Filippov A.A., Plotnikov I.S., Smurov A.O., Rusakova O.M., Zhakova L.V., Pirulin D.D. 1998. Living assiciationsof the northern part of the Aral Sea in 1993-1995. In: UNESCO (Ed.), Aral Sea Project 1992-1996, Final Scientific Reports. Paris. P. 95-138. Pichkily L.O. 1981. Fitoplankton Aralskogo morya v usloviyakh antropogennogo vozdeystviya (1957-1980). Kiev, Naukova Dumka. 228 pp. (in Russian). Zavialov P.O., Arashkevich E.G., Dikarev S.N., Kudyshkin T.V., Kurbaniyazov A.K., Ni A.A., Sapozhnikov F.V., Soloviev K.A., Khan V.M. 2006. Monitoring sostoyaniya fizicheskikh, khimicheskikh i biologicheskikh sistem Aralskogo Morya v usloviyakh ekologicheskogo krizisa. In: Sovremennye problemy aridnykh i semiaridnykh ekosistem yuga Rossii. Rostov-na-Donu. P. 529-562 (in Russian). 3. ARTEMIA POPULATION DYNAMICS IN THE PELAGIC ZONE OF THE ARAL SEA IN THE PERIOD 2005-2008 West females West gravid females East females East gravid females density (individuals/liter) 0.5 0.4 0.3 0.2 0.1 0 Aug-04 Feb-05 Sep-05 Mar-06 month-year 13 Oct-06 Apr-07 Nov-07 West Aral East Aral 18 cyst count per liter 16 14 12 10 8 6 4 2 0 Aug-04 Feb-05 Sep-05 Mar-06 Oct-06 Apr-07 Nov-07 month-year 4. EVOLUTION OF NON-ARTEMIA ZOOPLANKTON IN THE ARAL SEA IN THE PERIOD 2005-2008 4.1.Introduction Until the 1970’s, the composition of zooplankton in the Aral Sea was stable, comprising over 40 species in pelagial zones (Anonymous, 1974). The basis of the zooplankton fauna was formed by Arctodiaptomus salinus, Ceriodaphnia reticulata, Moina salina, Podonidae. In the 1960’s a copepod Calanipeda aquaedulcis Kritchagin was released into the Aral Sea. In the 1970-80’s this species was dominant in the zooplankton of the Aral Sea, which resulted in the disappearance of the former dominants, A. salinus, C. reticulata, M. salina (Aladin and Andreev, 1984). A drop in the inflow of the rivers and progressive salinization of the waters of the Aral Sea produced an adverse impact on the freshwater and brackish-water species, and they quickly vanished from the fauna. A quick decrease in the biodiversity of zooplankton was recorded in the first half of the 1970’s. By 1976, the species composition became stable as the average salinity of the Aral Sea reached 14 ppt (Andreev, 1989). Later, a gradual decrease in the zooplankton diversity took place (Table 4.1). Since 1997, the former dominant, Calanipeda aquaedulcis, vanished from the plankton, which was apparently the reason for the emergence of Moina salina and parthenogenetic Artemia in the plankton (Mirabdullayev et al., 2004). Data on Aral Sea zooplankton are abundant for the period till the 1980’s. As since 1981 fisheries collapsed on the Big Aral, data on its ecology since this period are very fragmentary. However, fast salinization of the water body over the last 20 years has caused fast changes in its biota. The goal of this study is to present recent data on the abundance of zooplankton, most of which species are food competitors for Artemia. 4.2. Material and Methods Samples were collected with a conical plankton net (diameter 36 cm), hauled from the bottom to the surface, and were fixed with formaldehyde until a final concentration of 2%. Zooplankton organisms were counted in a Bogorov counting chamber under a stereozoom microscope. In totally 132 samples were collected and processed. 14 Table 4.1. Succession of species composition of zooplankton of the Aral Sea during its transition from oligohaline to a hypersaline state (+ = present; - = absent) Taxa 1971 1981 1989 1994 2000 2005 2006 2007 Hexarthra fennica + + + – + + + – Brachionus plicatilis + + – – – + + – Nereis diversicolor + + + + + – – – Cerastoderma isthmicum + + + + + – – – Syndosmya segmentum + + + + + – – – Artemia sp. – – – – + + + + Moina salina + – + – + + + – Podonevadne camptonyx + + + – – – – – Apocyclops dengizicus + + + – – + – – Cletocamptus retrogressus + + + + + + + + Baeotendipes cf. noctivaga + + + + + + + + Total number of species 43 18 13 9 8 7 6 3 salinity (g. l-1) 12 18 30 37 58-63 68113 70136 >100 4.3. Results In May 2005 only 2 species of zooplankton were recorded in the West Aral, near Cape Kabanbay. In August 2005 4 species were recorded in the West Aral, with the rotiferan Hexarthra fennica as dominant speices, and 2 species in the East Aral with the copepod Apocyclops dengizicus as dominant. Zooplankton biomass was relatively low in May and August 2005 (Table 4.2). In September 2005 a big amount of water was discharged into the south part of the East Aral introducing nutrients and resulting in a decreased salinity and an increased bioproductivity. As a consequence, zooplankton species diversity and biomass increased sharply in the East Aral (Table 4.2). Brachionus plicatilis and Moina salina became dominants, species which had not been recorded over the last years. Table 4.2. Species composition and density of zooplankton in the Aral Sea in 2005 (D = dominant species) West Aral Turbellaria gen. sp. Hexarthra fennica Brachionus plicatilis Clethocamptus retrogressus Apocyclops dengizicus Moina salina Baeotendipes cf. noctivaga number of individuals/m³ biomass (mg/m³) May + D + 3,024 1.67 15 July September + D D + + + D + + + + 33,294 2,877 1.61 3.64 East Aral July + D + 144 2.07 September + + D + D + 48,654 609.23 In 2006 species diversity and quantitative development of zooplankton in the West Aral was extremely low, with the harpacticoid Cletocamptus retrogressus as the most constant representative (Tables 4.3 and 4.4). In the East Aral in April and May 2006 the diversity of zooplankton was comparatively high with Hexarthra fennica as a dominant species and with the appearance of the calanoid Arctodiaptomus salinus, a species not recorded in the Big Aral over about 20 years (Table 4.1). However in June 2006 the diversity and development of zooplankton decreased sharply, which is probably caused by the drop of inflow of water and the increased salinity. Table 4.3. Species composition of zooplankton in the Aral Sea in 2006 Turbellaria gen. sp. Brachionus plicatilis Hexarthra fennica Moina salina Arctodiaptomus salinus Cletocamptus retrogressus Blaeotendipes cf. noctivaga West Aral Apr May Jun Aug + + + + + + + + Sep + + Apr + + D + + + + May + + D + + + - East Aral Jun + + + Aug + + + Sep + + + Table. 4.4. Zooplankton density in Aral Sea in 2006 April number of individuals/m3 biomass (mg/m3) 0 0 number of individuals/m3 biomass (mg/m3) 53,250 18.56 May West Aral 484 3.28 East Aral 52,157 24.44 June August September 97 11.11 7 7.1 2 0.05 37 1.10 136 136.5 15 0.76 In 2007 only 3 species of zooplankton were recorded in the West Aral. No zooplankton was recorded in the East Aral due too high salinity (265 ppt). Zooplankton density was very low and less than in 2006 (Table 4.5). Table 4.5. Species composition and density of zooplankton in the Aral Sea in 2007 Turbellaria Cletocamptus retrogressus Baeotendipes cf. noctivaga number of individuals/m3 biomass (mg/m3) June + + 11.2 0.37 July + + 12.35 0.39 August + + + 1.77 0.06 September + + 0 0 4.4. Discussion Over the last five years, four species have represented almost permanent elements of the Big Aral Sea zooplankton: the rotiferan Hexarthra fennica, the branchiopod crustacean Artemia, the harpacticoid copepod crustacean Cletocamptus retrogressus and larvae of the dipteran chironomid Baeotendipes 16 cf. noctivaga (Table 1). Surprisingly, in 2004-2005 the cyclopoid Apocyclops dengizicus was recorded, a pecies which had not been observed since the mid-1990’s. There is a strong overall tendency towards decreased zooplankton species diversity.. However, sharp fluctuations in water inflow into the shallow East Big Aral in 2005-2006 resulted in considerable changes in the structure and composition of the zooplankton fauna: a fast salinity drop in the East Big Aral Sea (from 100 to 70 ppt) in autumn 2005 - spring 2006 and the inflow of nutrients resulted in a marked increase in zooplankton diversity and biomass (Tables 4.2 and 4.3) and in the appearance of such species as the rotiferan Brachionus plicatilis, the cladoceran Moina salina, and the calanoid copepod Arctodiaptomus salinus, which had not been recorded in the Big Aral in previous few years Later onwards biodiversity and biomass fell back on their previous low values (Table 4.5). The overall biomass densities, recorded during this sampling campaig, suggest that the development of non-Artemia zooplankton is too low to play a significant role in competition with the Artemia population, which is in accordance with the opinion expressed by Zavialov et al. (2006). Literature References Aladin, N.V.;Andreev, N.I. 1984. Vliyanie solenosti Aralskogo morya na izmenenie sostava fauni vetvistousikh rakoobraznikh. Gidrobiologicheskiy Zhurnal. V. 20. N3. P. 23-28 (in Russian). Andreev, N.I. 1989. The zooplankton of the Aral Sea during its initial period of salinisation. Proceed. Zool. Inst. USSR Acad. Sci. V. 199. P. 26-51 (in Russian). Anonymous. 1974.. Atlas of the invertebrates of the Aral Sea. (Ed. F.D. Mordukhay-Boltovskoy). M.: Pishevaya promyshlennost. 272 p. (in Russian). Mirabdullayev I.M., Joldasova I.M., Mustafaeva Z.A., Kazakhbaev S., Lyubimova S.A., Tashmukhamedov B.A. 2004. Succession of the ecosystems of the Aral Sea during its transition from oligohaline to polyhaline waterbody. J. Marine Syst. V. 47. N 1-4. P. 101-107. Zavialov P.O., Arashkevich E.G., Dikarev S.N., Kudyshkin T.V., Kurbaniyazov A.K., Ni A.A., Sapozhnikov F.V., Soloviev K.A., Khan V.M. 2006. Monitoring sostoyaniya fizicheskikh, khimicheskikh i biologicheskikh sistem Aralskogo Morya v usloviyakh ekologicheskogo krizisa. In: Sovremennye problemy aridnykh i semiaridnykh ekosistem yuga Rossii. Rostov- na-Donu. 17 PART 2. LABORATORY STUDIES 1. STUDY OF PHYTOPLANKTON SPECIES FROM THE ARAL SEA SEM investigations of frustules morphology of numerically dominant diatom species (Bacillariophyceae) from Aral Sea were performed Detailed SEM morphological analysis was carried out for some Cyclotella, Actinocyclus, Melosira, Cocconeis, Amphora, Navicula, Nitzschia, Surirella, and Campylodiscus.. A series of microalgal species were isolated from Aral samples available and introduced in monoclonal cultures. Representatives of genera Oocystis, Tetraselmis, Navicula, Nitzschia, Hantzschia, Mastogloia and Surirella proved to be cultivatable. Oocystis and Tetraselmis cultures were used in Artemia feeding experiments (see 2). Two diatom species (Fig. 2.1.) cultured were studied in more detail. Nitzschia sigma is one of the Nitzschia spp. deposited in the diatom culture collection of the PAE UGEnt (Chepurnov et al., 2008). The cell- and life-cycle of this diatom were studied experimentally; the mating system is heterothallic. Sexual reproduction was induced experimentally in monoclonal cultures of Surirella fastuosa; in addition this diatom exhibited interesting morphological feature as heterovalvy (a peculiar variation in structure of the siliceous exoskeleton of a single cell) – this was also studied in detail with the aid of scanning electron microscopy. Stomach contents of adult Artemia specimens (collected in Aral) were investigated too; microscopical analysis showed that Oocystis spp. were dominating in the diet of Artemia, but diatoms of the genera Cocconeis and Nitzschia were present in the contents as well. Fig. 2.1. Microscopic images of Diatoms (Bacillariophyceae) from the Aral Sea: Nitzschia o (A) Monoclonal culture o (B) Reproductive stage (auxospore) Surirella o (C) Siliceous part of cell wall, the frustule: light microscopy o (D) scanning electron microscope 18 2. STUDY OF THE CHARACTERISTICS OF THE PARTHENOGENETIC ARAL ARTEMIA STRAIN 2.1. Objectives The strain characteristics of the parthenogenetic Aral Sea Artemia were studied in a series of laboratory tests. These tests had a double objective: a) to study the suitability of the strain for larviculture applications; for this purpose, biometrics enrichment behaviour were studied; and b) to study the Artemia biology (survival and fecundity) in standardized laboratory conditions, and relate these to the observations on the population, made in the field. 2.2. Biometric characteristics 2.2.1. Material and Methods The cyst diameter was obtained by incubating a small sample of full cysts in 10 ppt seawater, add 2% lugol and keeping the sample overnight in darkness (Lavens and Sorgeloos, 1996). The cyst diameter of 100 cysts was measured with a microscope, equipped with a calibrated eyepiece. For determination of naupliar length, a small cyst sample was incubated in 35ppt seawater. After hatching, 100 (instar I) nauplii were measured (from tip of head until tip of telson) under a microscope. For determination of the hatching quality, a cyst sample of 1.6 g was incubated in 800 ml of 32 ppt artificial seawater (Instant Ocean) under continuous illumination (2000lux) at 28°C in a cylindroconical tube, provided with aeration to keep the cysts in suspension, and run in triplicate. After 24 h of incubation 6 samples of 250µl were taken from each cone, fixed using lugol solution and the nauplii and umbrellae were counted under a microscope. Unhatched cysts were decapsulated by adding NaOCl to each sample. The unhatched embryos were counted and the hatching percentage (H %) was calculated as follows: H% = (nauplii/nauplii+umbrellae+ embryos) * 100 2.2.2. Results Cyst diameter of the various samples analysed ranged between 249.4.5 -260.0 µm, but was in the order of 250-252 µm for most of the nine Aral samples analysed, a rather small value for a parthenogenetic strain, but comparable to the diameter of the commercially important Great Salt Lake Artemia (Vanhaecke and Sorgeloos, 1980). Instar I length varied between 493.6-499.5 µm. The hatching percentage of the respective samples varied between 10.0 and 93.5 %. The quantities of the samples were either too small, or the hatching was too high to allow a detailed study of the diapause requirements of the Aral Sea strain. 2. 3. Enrichment of Aral Sea Artemia nauplii 2.3.1. Objectives In order to evaluate the potential use of Aral Sea Artemia for larviculture applications, nauplii of the sample with ARC code number 1638 were hatched in standard conditions (Lavens and Sorgeloos, 1996), and enriched with a standard enrichment emulsion, the metabolic fate of which was followed during a subsequent starvation experiment. 2.3.2. Material and methods For sake of comparison with literature data, the standard enrichment emulsion “ICES50” (50% total n-3 HUFA on dry weight basis; DHA/EPA ratio of 0.87) was used. Temperature and salinity during enrichment were 28°C and 32g l-1. The enrichment was performed in triplicate 1 liter cones (800 ml water volume). The enrichment emulsion was administered at a dose of 0.2g l-1 at 0 and 12 h after hatching, i.e. at t0 and t12. Samples were harvested at t0, t12, and t24 for FAME analysis. After 24 h enrichment period the surviving Artemia nauplii were gently rinsed with seawater on a sieve to remove all residual emulsion, and then transferred to cylindroconical tubes at a density of 125 10ml1 , kept in a water bath at 28°C, for a subsequent starvation during 36 h. Samples were then taken after 19 12, 24 and 36 h of starvation (t36, t48 and t60). 2.3.3. Results and Discussion The EPA and DHA level of the ICES50 emulsion was 205.20 and 178.00 mg/g, respectively. The HUFA levels of the the non-enriched nauplii (t0), the enriched (t12 and t24) and the starved metanauplii (t36, t48 and t60) are shown in Figure 2.2. Absolute changes in HUFA contents following enrichment and starvation mg/g dry weight of Artemia 40 30 EPA DHA 20 > (n-3) > (n-6) 10 0 0 12 24 36 48 60 time (h) Fig. 2.2. Changes in HUFA contents (mg/g DW) in Aral Sea Artemia enriched for 12 h with emulsion ICES50, and during a subsequent 36 h starvation period As in Great Salt Lake A. franciscana (Han et al., 2001), parthenogenetic Aral Sea Artemia shows a differential accumulation of fatty acids during the enrichment period, followed by a differential breaking down during the starvation period. The exact levels of accumulation depend on various factors such as a) initial concentrations in non-enriched nauplii; b) composition of enrichment emulsion; c) specific conditions, such as temperature during enrichment and exact age of nauplii at the onset of the enrichment period. Nevertheless, the results show that comparable HUFA levels can be obtained with Aral Sea Artemia, as with the commercial GSL Artemia (Han et al., 2000; 2001). 2. 4. Effect of culture conditions on survival and fecundity of Aral Sea Artemia 2.4.1. Objectives A series of laboratory culture tests was set up in order to evaluate the effect of different culture conditions on the survival and reproduction of Aral Sea Artemia. The following culture parameters were analyzed: culture salinity culture temperature phytoplankton species (monodiet) applied (in comparison with a reference phytoplankton diet) density of phytoplankton cells applied: as the phytoplankton density of Aral Sea is low, different microalgae cells were tested, lower than the reference density used for standard Artemia culture (Lavens and Sorgeloos, 1996). 2.4.2. Material and methods 20 2.4.2.1. Cyst sample The Aral cyst sample with ARC code number 1638 (harvesting date: February 2005) was used throughouth the culture experiments. Its hatching percentage was 90.5 % ± 0.2 upon arrival at ARC (March 2005). 2.4.2.2. Algae species Oocystis sp. (Fig. 2.3) and Nautococcus sp. (Fig. 2.4) were isolated from Aral Sea water samples; Tetraselmis suecica was applied as reference diet for sake of comparison with literature data (Coutteau et al., 1992). The different algae were mass-cultured under standard conditions (Lavens and Sorgeloos, 1996) and the algae suspension was harvested and centrifuged in the exponential growth phase. The cell concentration of the algal suspension was counted using the Burker counting chamber, and the suspension was stored at 4°C and used for a maximum of 5 days (after which a new culture was ready for harvest). For cell size and FAME analysis of these 3 algal species, see Tables 2.1 and 2.2, respectively. Fig. 2.3. Microscopical view of Oocystis cells Fig. 2.4. Microscopical view of Nautococcus cells Table 2.1. Size of the individual algal cells (n=100) Species Length (µm) Width (µm) Tetraselmis suecica 7.31 ± 0.66 4.29 ± 0.72 Nautococcus sp. 12.05 ± 1.3 9.08 ± 1.16 Oocystis sp. 5.08 ± 1.07 2.54 ± 0.61 * determined according to Lavens and Sorgeloos (1996) Table 2.2. FAME –analysis in mg/g DW (3 replicates) of algal cells Tetraselmis Nautococcus 16:0 23.6 ± 0.2 38.5 ± 1.0 16:1(n-7) 2.6 ± 0.0 10.2 ± 0.3 18:1(n-9) 8.3 ± 0.1 50.2 ± 1.0 18:1(n-7) 2.8 ± 0.0 3.9 ± 0.1 18:2(n-6)-c 5.1 ± 1.0 13.2 ± 0.3 18:3(n-3) 22.0 ± 0.3 9.3 ± 0.2 18:4(n-3) 11.2 ± 0.1 2.3 ± 0.1 20:5(n-3) 7.6 ± 0.1 2.8 ± 0.1 22:6(n-3) 0.0 ± 0.0 0.0 ± 0.0 Sum(n-3) >or= 20:3(n-3) 8.1 ± 0.1 3.0 ± 0.1 Sum(n-6) >or= 18:2(n-6)-t 7.2 ± 0.1 16.5 ± 1.5 21 Biomass* (pg dry weight /cell) 153.90 ± 7.88 229.53 ± 7.78 283.75 ± 7.17 Oocystis 30.0 ± 3.4 5.9 ± 0.0 9.1 ± 0.6 2.9 ± 0.3 11.4 ± 0.4 19.4 ± 1.9 11.7 ± 1.1 6.6 ± 0.6 0.0 ± 0.0 7.2 ± 0.6 13.0 ± 0.5 Total mg FAME/g DW 115.3 ± 1.3 160.4 ± 4.6 129.1 ± 10.2 2.4.2.3. Feeding regimes Different feeding regimes (= number of algal cells supplied per Artemia individual) were applied, based on the feeding regime described by Coutteau et al. (1992) for Tetraselmis suecica, which was used as reference regime (“regime 1”) (Table 2.3). Other feeding regimes were a fraction (in the range ½ - 1/16) of the reference regime. Table 2.3. Feeding regimes applied (x 106 cells per Artemia individual) age of Artemia (days) 1 1/2 1/4 1/8 1/16 1 0.250 0.125 0.065 0.033 0.016 2,3,4 0.500 0.250 0.125 0.065 0.031 5,6 0.750 0.380 0.190 0.095 0.048 7 0.990 0.500 0.250 0.125 0.063 8 1.260 0.630 0.315 0.158 1.079 9 2.040 1.020 0.510 0.255 0.128 10,11 2.400 1.200 0.600 0.300 0.150 12,13 3.000 1.500 0.750 0.375 0.188 14,15 3.600 1.800 0.900 0.450 0.225 16,17 4.200 2.100 1.050 0.525 0.263 18,19 5.100 2.550 1.275 0.638 0.319 from 20 onwards 6.000 3.000 1.500 0.750 0.375 2.4.2.4. Experimental procedure The experimental procedure implied hatching of the cysts in standard hatching conditions (Lavens and Sorgeloos, 1996) and harvesting the nauplii after 24 hours. For each treatment (see further), 3 replicates of 500 instar I nauplii, incubated in one-liter culture cones were set up. Culture temperature was 22.0 or 28.0 ± 0.5°C. Culture medium was Instant Ocean artificial seawater solution (renewed 3 times weekly) of 60 or 120 g/l. Survival was monitored at each water renewal. At sexual maturity 50 specimens were isolated individually in 50 ml Falcon tubes (kept at the same salinity, temperature, feeding regime as before isolation). At each water renewal (3 times weekly) survival and fecundity (offspring per brood, number of broods per female, total offspring per female, percentage encystment) were observed until day 54 post-hatching. 2.4.2.5. Experimental treatments For the combinations of culture salinity, temperature and feeding conditions (phytoplankton species + feeding regime), see Table 2.4. Table 2.4. Experimental treatments Algal species Feeding regime Tetraselmis Nautococcus Oocystis 1 1/2 1/4 1/8 1/16 1 1/2 1/4 1/8 1/16 1 60 g/l x x - culture temperature and salinity 22°C 28°C 120 g/l 60 g/l x x x x x x x x x x x x x 22 120 g/l x x x x x x x x 1/2 1/4 1/8 1/16 - x - x x x x x - 2.4.3. Results and discussion 2.4.3.1. Survival Figs. 2.5, 2.6 and 2.7 show the survival of the culture tests run with Tetraselmis, Nautococcus and Oocystis, respectively. Results are shown for survival before (average of 3 cones) and after isolation (Figures A and B, respectively); red lines correspond with 28°C; blue lines with 22°C; full lines with 120 g/l and dotted lines with 60 g/l; different symbols correspond with different cell densities. Tetraselmis 100 Fig. 2.5.A. Tetraselmis before isolation 80 120 g/l - 1 - 28°C survival (%) 120 g/l - ½ - 28°C 60 60 g/l - 1 - 28°C 60 g/l - ½ - 28°C 120 g/l - 1 - 22°C 40 120 g/l - ½ - 22°C 60 g/l - 1 - 22°C 60 g/l - ½ - 22°C 20 age (days) 0 0 5 10 15 23 20 100 Fig. 2.5.B.Tetraselmis after isolation 80 120 g/l - 1 - 28°C 120 g/l - ½ - 28°C survival (%) 60 g/l - 1 - 28°C 60 60 g/l - ½ - 28°C 120 g/l - 1 - 22°C 120 g/l - ½ - 22°C 60 g/l - 1- 22°C 60 g/l - ½ - 22°C 40 age (days) 20 20 25 30 35 40 45 50 55 60 Fig. 2.5. Survival of Aral Sea Artemia on Tetraselmis diet, using different feeding rates and culture temperature and salinity before (2.5.A) and after (2.5.B) isolation of the individuals in separate culture vials Prior to isolation, highest mortality occurred within the first 10 days of culture. Final survival was higher at 120 g/l (range 20-29 %) than at 60 g/l (range 5-11 %). There were no clear trends for the other parameters. After isolation, survival generally gradually decreased to values inbetween 76-78 % (60 g/l – feeding ‘1’ – 22°C and 60 g/l – feeding ‘1/2’ – 28°C) and 54-56 % (120 g/l – ½ -22°C; 120 g/l – 1 – 28°C). Again no clear trends showed up. Nautococcus 24 100 Fig. 2.6.A. Nautococcus before isolation 120 g/l – 1 - 28°C 80 120 g/l – ½ - 28°C 120 g/l – ¼ - 28°C 120 g/l – 1/8 - 28°C survival (%) 60 120 g/l – 1/16 - 28°C 60 g/l - 1 - 28°C 60 g/l – ½ - 28°C 60 g/l – ¼ - 28°C 40 60 g/l – 1/8 - 28°C 60 g/l – 1/16 - 28°C 120 g/l – 1 - 22°C 20 120 g/l – ½ - 22°C age (days) 0 0 5 10 15 20 100 Fig. 2.6.B. Nautococcus after isolation 120 g/l – 1 - 28°C 80 120 g/l – ½ - 28°C 120 g/l – ¼ - 28°C survival (%) 120 g/l – 1/8 - 28°C 120 g/l – 1/16 - 28°C 60 g/l – 1 - 28°C 60 60 g/l – ½ - 28°C 60 g/l – ¼ - 28°C 60 g/l – 1/8 - 28°C 60 g/l – 1/16 - 28°C 40 120 g/l – 1 - 22°C 120 g/l – ½ - 22°C age (days) 20 20 25 30 35 40 45 50 55 60 Fig. 2.6. Survival of Aral Sea Artemia on Nautococcus diet, using different feeding rates and culture temperature and salinity before (2.6.A) and after (2.6.B) isolation of the individuals in separate culture vials Survival before isolation was often higher when Nautococcus had been fed, as compared to Tetraselmis (survival values inbetween 12 and 73 %). There was a general trend for lower survival when lower feeding rates were applied, both at 120 g/l and at 60 g/l. After isolation final survival varied between 36 and 90 %. During this culture phase, however, lower feeding rates resulted in higher final survival for 60 g/l, whereas there was no link between both factors at 120 g/l.. None of 25 both salinities performed better than the other. Oocystis 100 Fig. 2.7.A. Oocystis before isolation 80 120 g/l - 1 - 28°C 120 g/l - ½ - 28°C survival (%) 60 60 g/l - 1 - 28°C 60 g/l - ½ - 28°C 60 g/l – ¼ - 28°C 60 g/l – 1/8 - 28°C 40 60 g/l – 1/16 - 28°C 120 g/l - 1 - 22°C 120 g/l - ½ - 22°C 20 age (days) 0 0 5 10 15 20 25 100 Fig. 2.7.B. Oocystis after isolation 80 120 g/l - 1 - 28°C 120 g/l - ½ - 28°C survival (%) 60 g/l - 1 - 28°C 60 g/l - ½ - 28°C 60 60 g/l – ¼ - 28°C 60 g/l – 1/8 -28°C 60 g/l – 1/16 - 28°C 120 g/l - 1 - 22°C 40 120 g/l - ½ - 22°C age (days) 20 20 25 30 35 40 45 50 55 60 Fig. 2.7.. Survival of Aral Sea Artemia on Oocystis diet, using different feeding rates and culture temperature and salinity before (2.7.A) and after (2.7.B) isolation of the individuals in separate culture vials When Oocystis was fed, survival before isolation ranged between 73 (120 g/l – 1 – 22°C) and 32% 26 (120 g/l – 1 – 28°C) (and was thus generally better than when Tetraselmis was fed). Lower temperature gave higher survival than higher salinity, and, as in Nautococcus lower feeding rates resulted in lower survival. After isolation, final survival ranged between 58 (60 g/l – 1/16 – 28°C) and 34% (120 g.l – 1 – 22°C). In contrast to the pre-isolation culture phase, there was no decreasing survival with decreasing feeding rate (as in Nautococcus, there was rather an opposite trend at 60 g/l). 2.4.3.2. Fecundity parameters Fecundity was expressed as average brood size (Fig. 2.8), average number of broods per female (Fig. 2.9), average total individual offspring (Fig. 2.10) and percentage encystment (Fig. 2.11) for the 50 females, as recorded over the post-isolation period. Fig. 2.8. average brood size Tetraselmis Nautococcus Oocystis 160 140 120 100 80 60 40 20 0 C C C C C C C C C C C C °C 2°C 8° 28 ° 28 ° 2 8° 28° 2° 22 ° 8 ° 28° 28° 28 ° 2 8° 22 2 -2 -2 – – 1 1 8 6 6 1 8 ½ ¼ 1 ½ ¼ / ½ / 1 1 1 1 ½ l – /l – l – /l – l – /l – 1/ 1/ l– l– l– – – – g/ g/ g/ – – g g/ g/l g/ g/l g g / g /l g l l 0 0 / 0 / 0 0 0 6 g g 60 60 60 60 60 12 12 12 12 12 12 0 2 0 60 1 -2 culture conditions Fig. 2.8. Average brood size of reproducing Aral Sea Artemia, cultured until the age of 54 days in different feeding, salinity and temperature conditions; bars (with standard error bars) represent average of values of 50 individual females 27 Fig 2.9. average individual number of broods Tetraselmis Nautococcus Oocystis 10 8 6 4 2 12 0 g 12 /l – 1 0 g/ l – 28 12 ½ °C 0 g/ l – 28 12 0 ¼ °C g 12 /l – - 28 0 1/ °C g/ 8 l– -2 8 1/ 16 °C -2 8° C 60 g/ l 60 – 1 g/ l – 28 60 ½ °C -2 g/ 60 l – ¼ 8°C g/ 60 l – 1 28 °C / g/ l– 81 / 28 ° 16 C -2 8° 12 C 0 g/ 12 l – 1 0 g/ l – 22 ½ °C -2 2° C 60 g/ 60 l – g/ 1 – l– 2 ½ 2 °C – 22 °C 0 culture conditions Fig. 2.9. Average individual number of broods of reproducing Aral Sea Artemia, cultured until the age of 54 days in different feeding, salinity and temperature conditions; bars (with standard error bars) represent average of values of 50 individual females Fig. 2.10. average total individual offspring Tetraselmis Nautococcus Oocystis 1200 1000 800 600 400 200 12 0 12 0 g/ l– 1 -2 g/ l 8 – 12 ½ °C 0 g 12 /l – 28 ° C 0 ¼ g 12 /l – - 28 °C 0 1/ g/ 8 l– -2 8 1/ 16 °C -2 8° C 60 g/ l 60 – 1 g/ l – 28 60 ½ °C g/ l – 28° 60 C ¼ g/ l – - 28 60 °C 1/ g/ 8 l– -2 8 1/ 1 6 °C -2 8° 12 C 0 g/ 12 l – 1 0 g/ l – 22 ½ °C -2 2° C 60 g/ l – 60 g/ 1 – l– 2 ½ 2 °C – 22 °C 0 culture conditions Fig. 2.10. Average (total individual offspring of reproducing Aral Sea Artemia, cultured until the age of 54 days in different feeding, salinity and temperature conditions; bars (with standard error bars) represent average of values of 50 individual females 28 Fig. 2.11. % encystment Tetraselmis Nautococcus Oocystis 100 80 60 40 20 12 0 g 12 /l – 1 0 -2 g/ l 8 – 12 ½ °C 0 g 12 /l – 28 ° C 0 ¼ g 12 /l – - 28 °C 0 1/ g/ 8 l– -2 8 1/ 16 °C -2 8° C 60 g/ l 60 – 1 g/ l – 28 60 ½ °C g/ l – 28° 60 C ¼ g/ l – - 28 60 °C 1/ g/ 8 l– -2 8 1/ 1 6 °C -2 8° 12 C 0 g/ 12 l – 1 0 g/ l – 22 ½ °C -2 2° C 60 g/ 60 l – 1 g/ l – – 22 ½ °C – 22 °C 0 culture conditions Fig. 2.11. Percentage encystment of reproducing Aral Sea Artemia, cultured until the age of 54 days in different feeding, salinity and temperature conditions; bars represent overall value for 50 individual females The following general conclusions can be drawn from the fecundity tests (Figs. 2.8. to 2.11): Effect of temperature: for those treatments where comparison is possible, culture at 22°C resulted in a a signifcantly higher average brood size, but significantly lower number of broods (p < 0.001), as compared to 28°C. No significant difference between both temperatures was found for the total number of offspring; Effect of salinty: where comparison is possible, lower culture salinity results in signifcantly higher (p< 0.001) fecundity (average brood size, number of broods and total number of offspring), which can be explained by the higher metabolic (osmoregulatory) costs inherent to a higher salinity environment; Effect of algal species: when comparison is possible, higher fecundity values (quantified as average brood size, individual number of broods and total individual offspring) are obtained by feeding Nautococcus then by feeding Tetraselmis. Fecundity values obtained with Oocystis are more variable, and can be superior to Nautococcus, inferior to Tetraselmis, or inbetween both species depending on the culture conditions (temperature and salinity), feeding rate and parameter studied; Effect of feeding rate: fecundity is negatively affected by lower feeding rates; from feeding rate ¼ onwards, a negative effect is observed on average brood size, number of broods and total number of offspring (p < 0.001) when feeding Nautococcus and Oocystis (low feeding rates were not applied for Tetraselmis). This trend is thus observed in spite of the considerable size differences between both algae species; Oviparity: higher ambient salinity during reproduction results in higher values for oviparity (which is generally linked with the degree of unstability or stressfulness of the environment). Other factors (higher or lower feeding rates, higher or lower temperature) did not result in a clear 29 trend towards either increased or decreased oviparity. The individual cellular dry weight (Table 2.1.) of the algal species used, allows to compare the fecundity parameters based as a function of the algal biomass supplied to each Artemia individual, where comparison is possible. At 60 g/l salinity and 28°C culture temperature (comparison for Nautococcus and Oocystis for 5 different feeding rates) feeding results in a higher brood size for Nautococcus (Fig. 2.12 A) and a similar number of broods (Fig. 2.12 B) than Oocystis, resulting in a higher total individual offspring for the former (Fig. 2.12 C), with for both species decreased values at the lower end of the feeding range. Percentage encystment shows no clear link with feeding rate or algal species (Fig. 2.12 D). Nautococcus Oocystis Nautococcus 120 80 40 0 0 0.5 1 1.5 8 6 4 2 0 2 0 feeding rate as a function of algal biomass 0.5 1 1.5 2 feeding rate as a function of algal biomass A. average brood size B. average number of broods Nautococcus Nautococcus Oocystis 1200 70 1000 60 % encystment total individual offspring Oocystis 10 average number of broods average brood size 160 800 600 400 200 Oocystis 50 40 30 20 10 0 0 0 0.5 1 1.5 2 0 feeding rate as a function of algal biomass 0.5 1 1.5 2 feeding rate as a function of algal biomass C. total individual offspring D. % encystment Fig. 2.12. Fecundity parameters (A. average brood size, B. average number of broods, C. total individual offpsring, D. % encystment) as a function of feeding rate, taking into account algal biomass (feeding rates are presented relative to ‘1’, i.e. feeding rate ‘1’ using Tetraselmis) Comparison for the three algal species is only possible for 2 feeding rates, at three salinitytemperature combinations (Fig. 2.13; only values for total individual offspring are shown). Tetraselmis results in the lowest total individual offspring, whereas the combination 60 g/l-28°C gives the highest fecundity of the salinity-temperature combinations where comparison is possible. 30 60 g/l 28°C 120 g/l - 28°C 1200 1200 1000 total individual offspring total individual offspring 1000 800 Tetraselmis 600 Nautococcus Oocystis 400 200 800 Tetraselmis 600 Nautococcus Oocystis 400 200 0 0 0 0.5 1 1.5 2 0 feeding rate as a function of algal biomass 0.5 1 1.5 2 feeding rate as a function of algal biomass A) 120 g/l-28°C B) 60 g/l-28°C 120 g/l - 22°C 1200 total individual offspring 1000 800 Tetraselmis 600 Nautococcus Oocystis 400 200 0 0 0.5 1 1.5 2 feeding rate as a function of algal biomass C) 120 g/l-22°C Fig. 2.13. Total individual offspring as a function of feeding rate, taking into account algal biomass (feeding rates are presented relative to ‘1’, i.e. feeding rate ‘1’ using Tetraselmis) for the three algal species tested, and at three salinity-temperature combinations: A) 120 g/l-28°C; B) 60 g/l-28°C; C) 120 g/l-22°C 2.4.4. Conclusions still to be worked out further once I have some insight into the field data The objectives of these laboratory tests were a) to provide information about the usability of Aral Sea Artemia strain for larviculture applications; b) to provide background information on the reproductive potential of the strain in controlled laboratory conditions, and to mirror these to the data recorded in the field. The results show that the parthenogenetic Artema strain is comparable with the commercially important A. franciscana Great Salt Lake strain in terms of cyst biometrics, HUFA levels and accumulation of HUFA’s post-enrichment. As samples of diapausing cysts could not be obtained, no information could be generated on the diapause characteristics (and techniques needed to terminate diapause) for this strain. The laboratory culture tests show a clear effect of the feeding conditions (phytoplankton species and feeding rate) on fecundity. The situation in the field (where Artemia feeds on a variety of phytoplankton species, on bacterial conglomerates and on organic detritus particles) is much more complex than the highly simplified laboratory trophic tests. Nevertheless it is clear that two of the common algal species in the Aral (Nautococcus sp. and Oocystis sp.), when fed as sole diet, result in a reproductive output of the Artemia population surpassing the laboratory reference Tetraselmis diet. It is equally clear that there is a pronounced feeding rate effect on fecundity. Comparing the labotory data with the data from the field, it is clear that the Artemia population in the Aral Sea is living and reproducing at concentrations of algal cells below the optimal concentration. 31 2.5. Predation by Aral Sea copepods on Artemia nauplii 2.5.1. Objectives Two series experiments with Arctodiaptomus salinus (Copepoda, Calanoida) and Apocyclops dengizicus (Copepoda, Cyclopoida) were conducted. Both species were found co-occuring with Artemia in the Aral Sea. The aim of these tests was to evaluate the predation pressure exerted by these copepods on the Artemia population. 2.5.2. Material and methods. Artemia cysts (collected in the Aral Sea) were incubated in standard hatching conditions (Lavens and Sorgeloos, 1996) for 24 h. Fifty instar I nauplii were introduced into a Petri dish filled with Aral Sea water diluted to 35 g/l salinity. One adult female of Arctodiaptomus salinus or Apocyclops dengizicus was added to each Petri dish. The experiments were run at a temperature of 24-26°C. The test was run in 10 replicates for each copepod species; after 24 h all Petri dishes were checked for the presence of Artemia individuals. 2.5.3. Results and conclusion Apparently no consumption of Artemia nauplii was observed during these experiments. The used methodology is identical with the methodology used by LIH for the estimation of the consumption of larvae of Culex pipiens (Diptera, Culicidae). According to the (limited) set-up of this experiment, A. salinus and A. dengizicus can thus not be considered as major predators on Artemia. References Chepurnov, V.A., Mann, D.G., von Dassow, P., Vanormelingen, P., Gillard, J., Inzé, D., Sabbe, K., Vyverman, W. 2008. In search of new tractable diatoms for experimental biology. BioEssays, in press. Coutteau, P., Brendonck, L., Lavens, P., Sorgeloos, P. 1992 The use of manipulated baker's yeast as an algal substitute for laboratory culture of Anostraca. Hydrobiologia 234:25-32. Han, K., Geurden, I., Sorgeloos, P. 2000. Comparison of docosahexaenoic acid (22:6n-3) levels in various Artemia strains during enrichment and subsequent starvation. J. World Aq. Soc. 31: 469-475. Han, K., Geurden, I.,, Sorgeloos, P. 2001. Fatty acid changes in enriched and subsequently starved Artemia franciscana nauplii enriched with different essential fatty acids. Aquaculture 199: 93-105. Lavens, P., Sorgeloos, P., 1996. Manual on the production and use of live food for aquaculture. FAO Fisheries Technical Paper No. 361, 295 p. Vanhaecke, P., Sorgeloos, P. 1980. International Study on Artemia. IV. The biometrics of Artemia strains from different geographical origin: p. 393-405. In: The brine shrimp Artemia. Vol. 3. Ecology, culturing, use in aquaculture. Persoone, G.; Sorgeloos, P.; Roels, 0.; Jaspers, E. (Eds). Universa Press, Wetteren, Belgium, 456 p. 32 5. IMPLEMENTATION OF RESULTS (2-4 pages) This chapter gives a status of the implementation of the results at the end of the Project and of the expectations for the immediate and longer term future. Special attention should be paid to the economic and industrial benefits (short and long term) for the country. Wherever possible, a cost-benefit analysis, based on the R&D findings, should be included (in EUR). 33 6. CONCLUSIONS (1-2 pages) Here, the overall conclusions of the SfP experience are given. The tangible consequences of NATO's funding in EUR of the Project for the research team, the participating institutions and, if appropriate, the nation, should be outlined. 34 ANNEXES Annex 1: List of collaborators (internal and external); enumerate under separate headings the collaborators who obtained an advanced degree (MSc, PhD) through co-operation with the Project. Project directors Patrick Sorgeloos (NPD) Iskandar Mirabdullayev (PPD) Co-directors Thomas Bosteels Brad Marden Gilbert Van Stappen Wim Vyverman Iliya Zholdasova Young Uzbek scientists Lola Abdullayeva Nodira Jumaniezova Ablatdyin Musaev Zuri Mustafaeva Svetlana Lyubimova Marina Oryol Rahmetulla Temirbekov Other NATO scientists involved Victor Chepurnov External NATO advisor Shane Bradt End users Haitmurat Abdurakhmanov A. Abdusattarov Ubbiniyaz Ashirbekov Polat Reimov Jarilkap Tursinbekov Rukhulla Kurbanov Ubaidulla Nudirkhanov Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Rozier 44, B9000 Gent, Belgium (ARC) Laboratory of Ichthyology and Hydrobiology, Institute of Zoology, Uzbek Academy of Sciences, Niyazov st. 1, Tashkent, 700095, Uzbekistan (LIH) Great Salt Lake Artemia Cooperative, 5859 N. Cottonwood Canyon Rd. Mt. Green, UT, USA INVE Technologies, Artemia Task Force, PO Box 1306, 598 W Clark St. Grantsville, UT, USA Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Rozier 44, B9000 Gent, Belgium Laboratory of Protistology & Aquatic Ecology, Department of Biology, Ghent University, Krijgslaan, 281-58, B9000 Gent, Belgium (LPAE) Institute of Bioecology of the Karakalpak Branch of the Uzbek Academy of Sciences; 41, Berdakh prospect, 742000, Nukus, Uzbekistan (IB) LIH LIH IB LIH IB IB IB LPAE University of New Hampshire, Durham, New Hampshire, USA Ministry of Economy, Republic of Karakalpakstan, 97 Dosnazarov St., 97 Nukus, 742000, Uzbekistan Scientific Research Center for Development of Fisheries, Chilanzar 10, 700123 Tashkent, Uzbekistan International Fund of Aral Rescue, Doslik guzari 111, Nukus, 742000, Karakalpakstan, Uzbekistan Goskompriroda-State Committee for Nature Protection of Karakalpakstan, 742000, Nukus Ul. Berdakh Gozary, Uzbekistan Community of Moinaq, Hakimiyat of Moinaq district, Republic Karakalpakstan, Moinaq 743500, Uzbekistan Scientific Research Center for Development of Fisheries, Tashkent, Uzbekistan International Fund of the Aral Sea, GEF Agency, Tashkent, 35 Nikolai Gorelkin Fakhritdin Shaunsiev Nagmet Aimbetov Bakhretdin Muradov Vladislav Talskikh Uzbekistan Hydrological Center, Cabinet of Ministers, Tashkent, Uzbekistan UNDP Project on Aral Sea Academy of Science Uzbekistan, Karakalpak Branch, Nukus Ministry of Economy Uzbekistan, Tashkent Hydrometeorological Center of Uzbekistan, Tashkent 36 Annex 2: List of publications resulting from the Project. List of presentations given to the scientific community during major conferences, as well as to the broader society via the press (attach photocopies of articles) and other media.. Publications What tentative titles can we further list here ???? Chepurnov, V.A., Mann, D.G., von Dassow, P., Vanormelingen, P., Gillard, J., Inzé, D., Sabbe, K., Vyverman, W. 2008. In search of new tractable diatoms for experimental biology. BioEssays, in press. Mirabdullayev I.M., Musaev A., Jumaniezova N.I. 2006. New data on zooplankton of the Aral Sea. – Proceedings of the Uzbekistan Research Centre on Fishery Development, Tashkent, Uzbekistan 110115 (in Russian) Musaev, A., Hankuliev, K., Marden, B., Jumaniezova, N.I., Abdullaeva L.I.,, Mirabdullaev, I.M. 2005. Dynamics of the Artemia population in the Aral Sea. Biodiversity of Uzbekistan – monitoring and exploitation (in Russian), p. 179-179. Van Stappen, G., Marden, B., Abullaeva, L., Musaev, A., Mirabdullaev, I.M., Sorgeloos, P. Survival and fecundity of parthenogenetic Artemia from the Aral Sea, Uzbekistan, as a function of culture temperature and salinity and feeding conditions. In prep. Presentations at conferences Abdullayeva L.N., Khalilov S. 2006. Development of green algae Nautococcus grandis Korsh (Chlorophyta) from the Aral Sea in laboratory culture. – In: Current problems in biology, ecology and soil sciences. Conference of the National University of Uzbekistan, Tashkent, Uzbekistan, 17-18 November, Book of Abstracts p. 6-7. (in Russian). Abdullayeva L.N., Mahieu C.., Van Stappen G.. 2006. Peculiarities of development of Artemia parthenogenetica from the Aral Sea in experimental conditions. – In: Current problems in biology, ecology and soil sciences. Conference of the National University of Uzbekistan, Tashkent, Uzbekistan. Book of Abstracts p. 50-51 (in Russian). Hankuliev, K. 2008. Comparative changes in the hydrochemistry of the Aral Sea, Uzbekistan (20022007) and Karabogaz-Gol, Turkmenistan (2000-2002) and th corresponding alterations in resident biota. 10th International Confernce on Salt Lake Research. Salt Lake City, USA, May 12-14. Jumaniezova N.I. 2006. Biological characteristics of Artemia parthenogenetica of the Aral Sea. – International Conference on Problems of rational use and conservation of biological resources of Southern Aral Sea area. Nukus, Uzbekistan, May 16-17. Book of Abstracts p. 115. Jumaniezova N.I., Mirabdullayev I.M. 2006. Growth, development, survival and fecundity of Artemia parthenogenetica of the Aral Sea – Proceedings of the Ferghana State University, Supplement, p. 83 (in Russian). Mirabdullayev I., Abdullayeva L., Musaev A. 2007. Succession of zooplankton in the Aral Sea. – Human and climate forcing of zooplankton populations. 4th International Zooplankton Symposium. 28 May-1 June, Hiroshima, Japan. Book of Abstracts, p. 216. Mirabdullayev I.M., Taizhanov E.B., Kuzmetov A.R., Urazova R.S. 2006. Aral Sea and problems of increasing of productivity in terminal irrigation lakes - In: Materials of the International Scientific Practical Conference ‘Urgent problems of ecology and nature use in Kazakhstan and adjacent territories”, Pavlodar, Kazakhstan, May 25-26, p. 345-347 (in Russian). 37 Mirabdullayev I.M., Urazova R.S., Taizhanov E.B. 2006. Use of Aral Sea hydrofauna for increasing of bioproductivity of waterbodies in Central Asia. – In: International Conference on Problems of rational use and conservation of biological resources of Southern Aral Sea area. Nukus, Uzbekistan, May 16-17, Book of Abstracts p. 16 (in Russian). Musaev A., Abdullayeva L.N. 2006. Influence of food concentration, salinity and temperature on survival of Artemia from the Aral Sea. – In: International Conference on Problems of rational use and conservation of biological resources of Southern Aral Sea area. Nukus, Uzbekistan, May 16-17, Book of Abstracts p. 117 (in Russian). Musaev A., Abdullayeva L.N., Jumaniezova N.I., Mirabdullayev I.M. 2007. Succession of zooplankton of the Aral Sea. – In: Conference on Problems of rational use of natural resources of Southern Aral Sea area. Nukus, Uzbekistan. Book of Abstracts p. 27 (in Russian). Mustafaeva Z.A., Abdullayeva L.N. 2007. New data on phytoplankton of the Aral Sea. – In: Biology of Inland waters. Reports of the XIII International Youth conference, Borok, Russia (in Russian), 2326 October, p. 45 (in Russian). Mustafaeva Z.A., Chepurnov V.A. 2006. К вопросу о систематическом положении диатомей родов Cymbella и Encyonema – In: Conference of the National University of Uzbekistan Current problems in biology, ecology and soil sciences. Tashkent, Uzbekistan, 17-18 November, Book of Abstracts p. 25 (in Russian). Mustafaeva Z.A., Abdullayeva L.N., Chepurnov V.A., Mirabdullayev I.M. 2006. Changes in plankton of the Aral Sea in conditions of progressing salinization – Proceedings of the Ferghana State University. Supplement, p. 79-80 (in Russian). Urazova R.S., Mirabdullayev I.M. Materials to revision of the genus Moina Baird, 1850 (Crustacea, Cladocera) in Uzbekistan. 2006. Conference of the Mamun Academy. Khiva, Uzbekistan, p. 189-191 (in Russian). Van Stappen, G. 2008. Artemia of the Aral Sea, Uzbekistan. Field survey of an emerging population and perspectives for commercial exploitation. 10th International Confernce on Salt Lake Research. Salt Lake City, USA, May 12-14. MSc Thesis Achom, H.O. 2008. Characterization of the parthenogenetic Artemia population of the Aral Sea, Uzbekistan. MSc Thesis, Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Belgium 38 Annex 3: Complete Inventory Record Serial Number Date of Purchase Cost USD Cost EUR Location TRX 500 FM TRX 500 FM TRX 500 FM TRX 500 FM IHFTE310854000655 20-Jun-05 IHFTE310754002204 20-Jun-05 IHFTE310554000676 20-Jun-05 IHFTE310154001128 20-Jun-05 5944,00 5944,00 5944,00 5944,00 4755.20 4755.20 4755.20 4755.20 IB IB IB IB YSI 4JE-221297 still to be registered 27-Jul-05 1536,00 1228.80 IB YSI 4JE-221298 still to be registered 18-Aug-05 1674,00 1339.20 IB Meiji A-4802-00 still to be registered 7-Jul-05 1995,00 1596.00 LIH Meiji A-48401-15 still to be registered 7-Jul-05 1910,00 1528.00 IB 2138.64 2138.64 IB IB Inventory Label No. No. A-0180 No. A-0181 No. A-0182 No. A-0183 Property Item Honda ATV Honda ATV Honda ATV Honda ATV Manufacturer Honda Honda Honda Honda No. A-0184 YSI Model 85 Oxygen, Salinity, Conductivity No. A-0185 & Temperature Meter (50 ft cable) YSI Model 85 Oxygen, Salinity, Conductivity Model Number & Temperature Meter (100 ft Cable) No. A-0186 Meiji Stereozoom Microscope System (7x to 45x zoom)(230VAC) No. A-0187 Meiji Professional Trinocular Compound Microscope with achromatic objective No. A-0188 No. A-0189 Caribe Rigid Inflatable Vessel Caribe Rigid Inflatable Vessel Caribe Inflatables C12/Light Grey EMD 12136G506 25-Jul-05 Caribe Inflatables C12/Light Grey 25-Jul-05 No. A-0176 DELL Notebook Computer DELL Computers Latitude X1 1-Sep-05 1839,83 1471.86 LIH No. A-0177 DELL Notebook Computer DELL Computers Latitude X1 1-Sep-05 1839,83 1471.86 LIH No. A-0178 DELL Notebook Computer DELL Computers Latitude X1 31-Aug-05 1839,83 1471.86 IB No. A-0179 DELL Notebook Computer DELL Computers Latitude X1 EMD 12137G506 CN-0P8056-3652158J-009A CN-0P8056-3652158J-0060 CN-0P8056-3652158J-008D CN-0P8056-3652158J-0099 2673,30 2673,30 31-Aug-05 1839,83 1471.86 IB 39 SUMMARY REPORT OF THE FINAL REPORT NPD: Patrick Sorgeloos, Laboratory of Aquaculture & Artemia Reference Center, Ghent University, Belgium PPD: Iskandar Mirabdullayev, Laboratory of Ichthyology and Hydrobiology, Institute of Zoology, Uzbek Academy of Sciences, Tashkent, Uzbekistan 1. Background and objectives The ecological devastation and economic impoverishment of the Aral Sea communities are threats to the security of the region and to the health and well-being of the people. There is an immediate need for the communities surrounding the Aral Sea to improve their quality of life through economic opportunities, to compensate for the loss of fish stocks upon which they formerly depended for their sustenance and employment. There is evidence of a recently colonizing population of the halophilic brine shrimp Artemia in the Aral Sea, that holds promise as a commercially viable resource: this crustacean is an essential and highly priced live food item in marine aquaculture worldwide. The development and prudent management of an Aral Sea Artemia resource could provide some degree of economic and ecological recovery to the region through sustainable management strategies. However, it is insufficiently known whether the current hydrobiological and hydrochemical status and primary productivity of the Aral Sea are sufficient to support a stable Artemia population. Detailed ecological information is needed in order to evaluate the feasibility and potential benefits of commercial exploitation of Artemia. Expert advice in harvesting, transport, handling, storage, and industrial production techniques are additionally essential for the successful exploitation of this resource. This project consequently aimed at providing the partner institutes and end-users with information on possible benefits and outcomes extending beyond the project’s duration: a) characterizing the Aral Sea Artemia population in terms of life history, population dynamics (and ecological conditons affecting them), cyst quality characteristics and potential for aquaculture; b) pending the outcome of (a) development of a population model for Aral Artemia resource and issue recommendations on optimal sustainable resource management; and c) transfer of technology (field and laboratory equipment) and know-how (extensive training programme). 2. Methodology 2.1. Field research Design of appropriate sampling campaign covering East and West Aral: sampling sites located along transsects; sampling frequency at least once a month in period April-October; Sampling for hydrochemistry (nutrients), other abiotic factors, phytoplankton composition and density, Artemia population composition and density, other zooplanktonic organisms. 2.2. Laboratory studies phytoplankton:, isolation, determination and culture of Aral Sea algal species at different abiotic conditions; Artemia: strain characteristics and cyst quality control: hatching and diapause behaviour, biometrics, nutritional profile; life history (growth, survival) and reproductive characteristics: effect of abiotic conditions temperature, salinity, feeding. 2.3. Training program for partner country (young) scientists Terms of reference: a) Ghent University, Belgium: algal isolation, determination and culture; hydrochemical analyis; Artemia biology and quality control; theory on harvesting and processing of Artemia cysts; b) INVE Thailand, Bangkok: Artemia cyst processing and storage; use of Artemia biomass in shrimp culture; c) Great Salt Lake Artemia Cooperative, Mountain Green, USA: industrial-scale harvesting and processing of Artemia cysts; Artemia resources management and modeling; d) at partner institutes in Uzbekistan: hands-on training in field sampling, sampling analysis and data processing. 40 3. Results 3.1. Field research The project was successful in covering the designed sampling programme (see picture) in the seasons 2005, 2006 and 2007, in the period March/April-October/November -depending on weather conditions. An additional sampling expedition was organized in December 2007 focusing on the nutrient contents of West Aral in deeper water layers. As compared to the start of the project, salinity in the West Aral has slowly increased from 70-80 to 80-90 ppt, and in the East Aral (with some occasional salinity drops due to increased freshwater inflow) from 90-100 to 120-130 ppt. This moderate salinity increase has, however, major consequences for the East Aral in terms of its potential for Artemia exploitation. Though this water body initially seemed more promising than the West Aral, thanks to its higher salinity and its relatively higher nutrient status (occasional Amu Darya water inflow), the further drop of the water level in the period 2005-2007 and the retreat of the shoreline have turned nearly the entire East Aral’s perimeter (on Uzbek territory and as far as publicly accessible) into extended mudflats practically inaccessible for any major vehicle (such as vessels or trucks for harvesting resp. transport of cysts). Moreover, the remaining water body, though still relatively large in surface, is basically a shallow (1.5- 2 m), nutrient-poor environment with low densities of Artemia. The West Aral, on the other hand, though more easily accessible (at least along its western shore) shows even lower nutrient contents, including in deeper water layers, and consequently very low phytoplankton and Artemia densities. The continuing uncertain situation regarding the future water management of the tributary rivers (a complex problem with many stakeholders involved – also at the international level) is a fundamental problem for any conceivable Aral Artemia exploitation plan. As there are no concrete short or medium-term indications that these basic impediments for Artemia exploitation are likely to alter in a positive sense, a commercially viable Artemia industry at West or East Aral thus seems unlikely in the foreseeable future. 3.2. Laboratory studies Laboratory studies were conducted as planned and show that the characteristics of the parthenogenetic Aral Sea Artemia strain, relevant for larviculture application (e.g. cyst biometrics, nutritional composition, diapause characteristics) are comparable to the Great Salt Lake strain. Reproduction tests show that the reproduction in the field is clearly negatively affected by the ambient low food levels. Nevertheless, even in optimal (laboratory) food conditions the reproductive capacity of the Aral Artemia strain is lower than in the commercially used A. franciscana. 3.3. Training program The training programme was executed as designed. 4. Implementation The data generated by the project have general generic value for possible Artemia exploitation issues and opportunities elsewhere in Uzbekistan. Further, implementation of the project’s results is ensured through the following channels: a) organization of workshops: 2 workshops for the end-users have been organized (i.e. one more than foreseen in the project proposal. The first workshop, organized November 7-8, 2005, Tashkent, aimed at informing the end-users about the goals and methodology of the project, and 41 b) c) d) e) at providing a forum for end-users feedback. The concluding workshop, organized November 5-6, 2007, sketched the overall results of the project to the end-users and drew outlines for future Artemia-related activities in Uzbekistan; development of critical mass of Artemia expertise and know-how at Uzbek partners institutes; purchase, transfer and installation of laboratory and field equipment at Uzbek partner institutes; Artemia- and aquaculture- related literature and documentation made available for Uzbek partner institutes; project-related publications: 13 publications in national or regional journals; XXX publications to be submitted to international peer-reviewed journals. 42