Molar mass by freezing point depression
advertisement
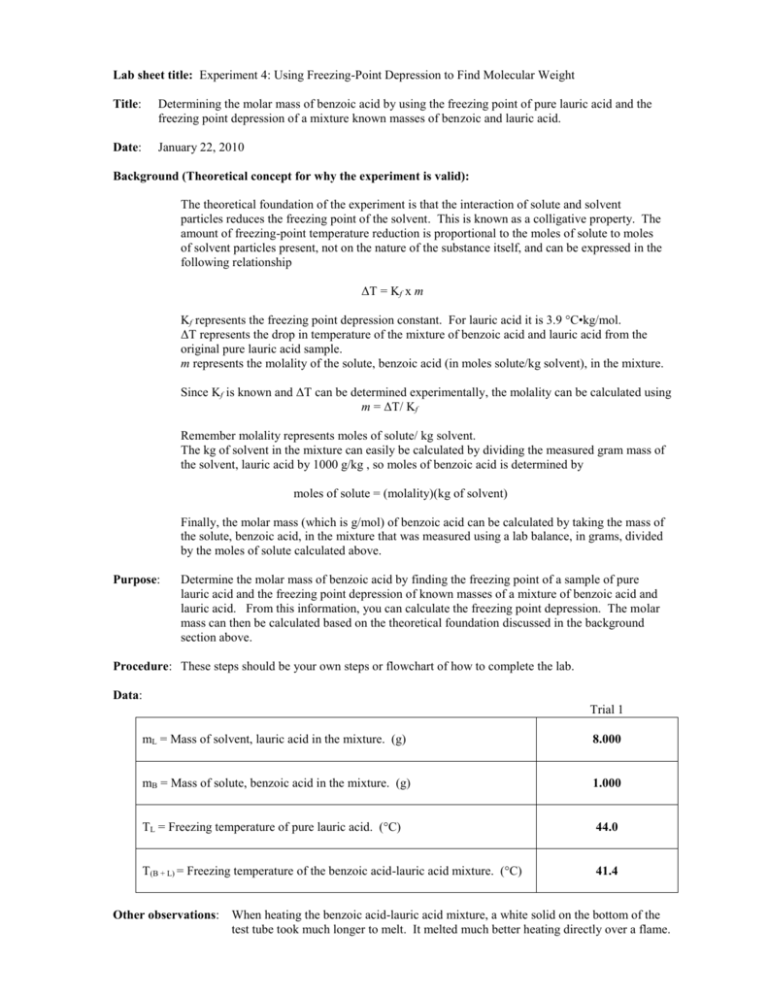
Lab sheet title: Experiment 4: Using Freezing-Point Depression to Find Molecular Weight Title: Determining the molar mass of benzoic acid by using the freezing point of pure lauric acid and the freezing point depression of a mixture known masses of benzoic and lauric acid. Date: January 22, 2010 Background (Theoretical concept for why the experiment is valid): The theoretical foundation of the experiment is that the interaction of solute and solvent particles reduces the freezing point of the solvent. This is known as a colligative property. The amount of freezing-point temperature reduction is proportional to the moles of solute to moles of solvent particles present, not on the nature of the substance itself, and can be expressed in the following relationship ΔT = Kf x m Kf represents the freezing point depression constant. For lauric acid it is 3.9 °C•kg/mol. ΔT represents the drop in temperature of the mixture of benzoic acid and lauric acid from the original pure lauric acid sample. m represents the molality of the solute, benzoic acid (in moles solute/kg solvent), in the mixture. Since Kf is known and ΔT can be determined experimentally, the molality can be calculated using m = ΔT/ Kf Remember molality represents moles of solute/ kg solvent. The kg of solvent in the mixture can easily be calculated by dividing the measured gram mass of the solvent, lauric acid by 1000 g/kg , so moles of benzoic acid is determined by moles of solute = (molality)(kg of solvent) Finally, the molar mass (which is g/mol) of benzoic acid can be calculated by taking the mass of the solute, benzoic acid, in the mixture that was measured using a lab balance, in grams, divided by the moles of solute calculated above. Purpose: Determine the molar mass of benzoic acid by finding the freezing point of a sample of pure lauric acid and the freezing point depression of known masses of a mixture of benzoic acid and lauric acid. From this information, you can calculate the freezing point depression. The molar mass can then be calculated based on the theoretical foundation discussed in the background section above. Procedure: These steps should be your own steps or flowchart of how to complete the lab. Data: Trial 1 mL = Mass of solvent, lauric acid in the mixture. (g) 8.000 mB = Mass of solute, benzoic acid in the mixture. (g) 1.000 TL = Freezing temperature of pure lauric acid. (°C) 44.0 T(B + L) = Freezing temperature of the benzoic acid-lauric acid mixture. (°C) 41.4 Other observations: When heating the benzoic acid-lauric acid mixture, a white solid on the bottom of the test tube took much longer to melt. It melted much better heating directly over a flame. Calculations: Freezing point depression of benzoic acid-lauric acid mixture ΔT = TL – T(B + L) ΔT = 44.0 °C – 40.4 °C = 3.6 °C Note Kf = 3.9 °C•kg/mol for lauric acid as a solvent. Molality of the mixture: m Kf m 3.6C moles benzoic acid 0.9231 m 0.92 3.9 C kg/mol kg lauric acid Mass, in kilograms, of lauric acid in the mixture mL = 8.000g lauric acid 1 kg 0.008000 kg lauric acid 1000 g Moles of the solute, benzoic acid, in the mixture moles of solute = (molality)(kg solvent) moles of solute = (0.9231 moles benzoic acid/kg lauric acid)(0.008000 kg lauric acid) moles of solute = 0.007385 moles benzoic acid = 0.0074 moles benzoic acid Molar mass of the solute, benzoic acid Molar mass = mB /moles of solute Molar mass = 1.000 g / 0.007385 moles Molar mass = 135.42 = 135.4 g/mol = 140 g/mol % Error Calculation Accepted molar mass of benzoic acid, C6H5COOH, = 122.0 g/mol Experimental molar mass of benzoic acid = 106.0 g/mol % error % error accepted value - experiment al value accepted value 122.0 g/mol - 135.4 g/mol 122.0 g/mol 100 100 % error = 11 % error Note: Final result represents no rounding for significant figures until the final answer. Conclusion: The molar mass of benzoic acid was experimentally determined to be 135.4 g/mol as compared to the accepted value of 122.0 g/mol resulting in 11% error. This value was calculated using the freezing point depression of a benzoic acid-lauric acid mixture and the relationship: ΔT = Kf x m. The lab results supported the theory that a solute-solvent mixture would lower the freezing point of the solvent. The lab also supported the idea that the molar mass of the solute can be determined as long as you know the freezing point depression constant of the solvent, mass data for the solute-solvent mixture as well as the temperature change of the pure solvent compared to the solute-solvent mixture. Error Analysis: The experimental value of molar mass for benzoic acid was 135.4 g/mol. The theoretical value of 122.0 g/mol for molar mass was calculated from the formula C6H5COOH for benzoic acid. This results in a relative error of 11 % as shown in the calculation section of the report. When calculating ΔT using the theoretical molar mass of benzoic acid and the mass values used in the lab, the ideal ΔT should have been 4.0 °C. The experiment showed a lower ΔT of 3.4 °C. The most likely reason for the 0.6°C lower ΔT than expected would come from the benzoic acid-lauric acid mixture not being completely mixed before starting the freezing process. Since there was a small amount of a white solid, probably the benzoic acid, which did not melt easily, it is quite possible that when the solid did finally melt it did not get mixed completely before beginning the freezing process. This would have resulted in less benzoic acid molecules being dispersed among the lauric acid molecules causing a less significant drop in freezing temperature. Another reason for the lower ΔT could be related to warming air in the room surrounding the thermometer instead of the solid. This could be the result of a large hole produced moving the thermometer up and down in the freezing process. Mass measurements were made on the analytical balance and it is unlikely that these measurements contributed much error in the lab. Post-Lab Questions from Lab Worksheet: (See calculation section for work) 1. The molality of the benzoic acid-lauric acid mixture was 0.92 m. 2. There were 0.0074 moles of benzoic acid solute in the benzoic acid-lauric acid mixture. 3. The experimental molecular weight of benzoic acid was 135.4 g/mol. 4. The accepted molecular weight of benzoic acid from its formula, C6H5COOH, is 122.0 g/mol. 5. The percent discrepancy between the experimental value of 135.4 g/mol and accepted value of 122.0 g/mol is 11% error. Lab 4 (Calculating Molar Mass using Freezing Point Depression) 2006 values Empty Test Tube (LA) Empty Test Tube (LA + BA) Lactic Acid Benzoic Acid 1 2 3 4 17.443 g 17.447g 17.477 g 17.282 g 17.425 g 7.998 g 1.012 g 17.392 g 7.965 g 1.004 g 17.426 g 7.985 g 0.999 g 17.391 g 8.007 g 1.004 g 5 6 8.002 g 1.003 g 7.999 g 1.005 g








