ΕΘΝΙΚΟ ΣΥΣΤΗΜΑ ΔΙΑΠΙΣΤΕΥΣΗΣ
advertisement
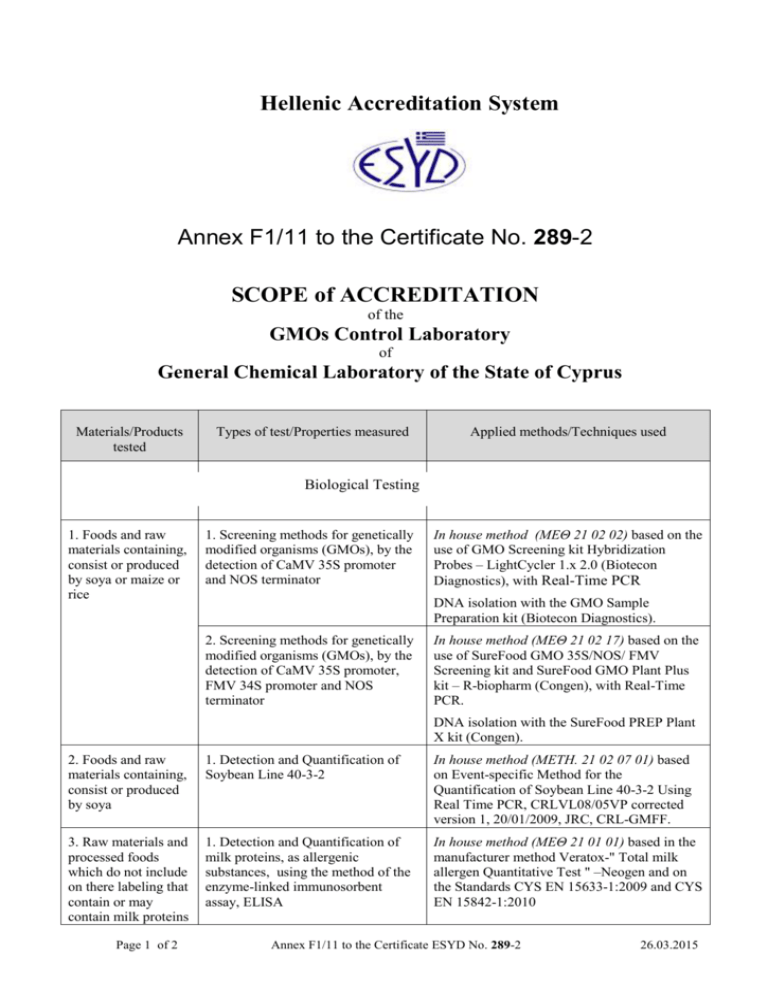
Hellenic Accreditation System Annex F1/11 to the Certificate No. 289-2 SCOPE of ACCREDITATION of the GMOs Control Laboratory of General Chemical Laboratory of the State of Cyprus Materials/Products tested Types of test/Properties measured Applied methods/Techniques used Biological Testing 1. Foods and raw materials containing, consist or produced by soya or maize or rice 1. Screening methods for genetically modified organisms (GMOs), by the detection of CaMV 35S promoter and NOS terminator In house method (ΜΕΘ 21 02 02) based on the use of GMO Screening kit Hybridization Probes – LightCycler 1.x 2.0 (Biotecon Diagnostics), with Real-Time PCR DNA isolation with the GMO Sample Preparation kit (Biotecon Diagnostics). 2. Screening methods for genetically modified organisms (GMOs), by the detection of CaMV 35S promoter, FMV 34S promoter and NOS terminator In house method (ΜΕΘ 21 02 17) based on the use of SureFood GMO 35S/NOS/ FMV Screening kit and SureFood GMO Plant Plus kit – R-biopharm (Congen), with Real-Time PCR. DNA isolation with the SureFood PREP Plant X kit (Congen). 2. Foods and raw materials containing, consist or produced by soya 1. Detection and Quantification of Soybean Line 40-3-2 In house method (METH. 21 02 07 01) based on Event-specific Method for the Quantification of Soybean Line 40-3-2 Using Real Time PCR, CRLVL08/05VP corrected version 1, 20/01/2009, JRC, CRL-GMFF. 3. Raw materials and processed foods which do not include on there labeling that contain or may contain milk proteins 1. Detection and Quantification of milk proteins, as allergenic substances, using the method of the enzyme-linked immunosorbent assay, ELISA In house method (ΜΕΘ 21 01 01) based in the manufacturer method Veratox-" Total milk allergen Quantitative Test " –Neogen and on the Standards CYS EN 15633-1:2009 and CYS EN 15842-1:2010 Page 1 of 2 Annex F1/11 to the Certificate ESYD No. 289-2 26.03.2015 Materials/Products tested Types of test/Properties measured Applied methods/Techniques used 4. Raw materials and processed foods which do not include on there labeling that contain or may contain almond proteins. 1. Detection and Quantification of almond protein, as allergenic substances, using the method of the enzyme-linked immunosorbent assay, ELISA In house method (ΜΕΘ 21 01 07) based in the manufacturer method Veratox "Quantitative Almond Allergen Test"- Neogen and on the Standards CYS EN15633-1:2009 and CYS EN15842:2010 5. Raw materials and processed foods which do not include on there labeling that contain or may contain peanut 1. Detection and quantification of peanut, as allergenic substance in foods, with the method of Enzymelinked immunosorbent assay (ELISA) In house method (METH 21 01 06 01) based on the manufacturer method (Biokits Peanut Assay Kits της εταιρίας Neogen) and on the 6. Raw materials and processed foods which do not include on there labeling that contain or may contain hazelnut 1. Detection and quantification of hazelnut, as allergenic substance in foods, with the method of Enzymelinked immunosorbent assay (ELISA) In house method (METH 21 01 08 03) based on the manufacturer method Ridascreen Fast Hazelnut-Enzyme immunoassay for the quantitative detection of Hazelnut - RBiopharm and on the Standards CYS EN15633-1:2009 and CYS EN15842:2010 Standards CYS EN15633-1:2009 and CYS EN15842:2010 Site of assessment: Permanent Laboratory premises, General Chemical Laboratory Branch, 39 Kyriakos Matsis Avenue, Agioi Omologites, 1082 Nicosia, Cyprus. Approved signatories: A. Varnava – Tello. This Scope of Accreditation replaces the previous one dated 27.11.2014. The Accreditation Certificate No. 289-2, to CYS EN ISO/IEC 17025:2005, has been expanded until 30.04.2015. Athens, March 26, 2015 Ioannis Sitaras Director of the Laboratories Accreditation Division Page 2 of 2 Annex F1/11 to the Certificate ESYD No. 289-2 26.03.2015