Service Technician - to the Pool Route Pros
advertisement

Swimming Pool & Spa Service This free copy is provided to you by: Training Guide 2nd Edition, March, 2010 Introduction Welcome to the Swimming Pool & Spa Service Training Guide. This guide is a tool to assist you, a swimming pool & spa service technician in providing the highest quality and most efficient swimming pool and spa service experience to your valued customers and a more efficient and safe service experience for you. This manual will cover service expectations in a chronological order from the time you gear up and start your truck to the time you end your day. This guide will cover such topics as service equipment, service techniques, equipment inspections, how to handle customer service in the field, basic and advanced chemistry techniques and much more. There will also be helpful and education notes located in gray boxes and caution notes in yellow boxes throughout this guide. You will see an example of Did you know? this in the gray box on the right. The two most common service reasons for a customer to cancel At the end of each chapter there will be a brief their swimming pool and spa review of that chapter with a summary of things to service are also the two easiest to remember. There will also be an accompanying prevent, algae growth and no multiple choice test at the end of this training shows. Don’t make the mistake guide to aid you in your service education and of allowing either one of these understanding of each topic. I believe that the issues to affect your customers. more educated a service technician is than the more confident and capable that technician will be in the field and that will reflect to the customer giving them the peace of mind that they deserve. *Disclaimer notice* This training guide is intended to be used as a guide for swimming pool and spa service technicians and is not intended to be used as a study guide for other service tests such as C.P.O. certification, water chemistry certification or any other service or chemistry tests. The views and policies of the writer may differ from those in other water chemistry manuals and are intended for us as a guide only. 1 Table of Contents i.) Introduction ……………………………………….……………………..… c.) Table of Contents ……………………………………………………...… 1.) Equipment Found on a Typical Pool Service Vehicle …………...….. 2.) Chemicals Commonly Found on a Pool Service Vehicle ……...……. 3.) Three Keys to Clear, Clean and Healthy Water ……………...………. 4.) About Chlorine, Sanitizers and Oxidizers ……………………..……… 5.) pH Balance (Power of Hydrogen) ……………………………………… 6.) Total Alkalinity (T.A.) ………………………......................................... 7.) Conditioner, Stabilizer (Cyanuric Acid) ………………………….…….. 8.) Calcium Hardness ……………………………………………..…….….. 9.) Algae! Know Your Enemy …………………………………………..….. 10.) Algaecides and Phosphate Removers ….……………………...……. 11.) Treatments for Algae ……………………………………………...…… 12.) Salt Chlorination Systems …………………………………………….. 13.) About Swimming Pool Filters ………………………………….……… 14.) Different Types of Pool and Spa Surfaces …………………….……. 15.) Types of Surface Staining …………………………………………….. 16.) A Typical Service Stop ……………………….................................... 17.) Common Problems Encountered in the Field …………………….… 18.) Examples of Chemicals Added at Typical Service Stops ………….. 2 1 2 3–5 6 7–8 9 – 17 18 – 19 20 – 21 22 – 23 24 – 25 26 – 28 29 – 33 34 – 51 52 – 57 58 – 62 63 – 68 69 70 – 72 73 – 76 77 – 81 82 – 85 86 – 90 91 – 95 96 97 – 99 100 – 101 102 – 112 113 – 138 139 – 142 143 19.) Desired Chemistry Levels Reference Chart ………………………… 20.) What is Expected of a Service Technician ……………………….…. 21.) Specialty Services (Chemical Only and Spa Service) …….............. 22.) How to Calculate a Pool’s Volume (Gallons) ……………………….. 23.) Safety Revisited ………………………………………......................... 24.) Additional Notes About the Pool Service Business ……..………….. 25.) Helpful Pool Service Forms and Documents ……………………….. 26.) Service Technician Training Guide Tests ……………..…………….. 27.) Glossary of Pool and Spa Service Terms …………………………… 28.) Final Notes from the Author ………………………………………...… 1. Equipment Found on a Typical Pool Service Vehicle The typical pool service vehicle can be equipped with a large variety of items, depending upon the service technician and his or her particular style. The following is a list of commonly used equipment and their descriptions to better understand the tools of the trade. Pole: The service technician’s pole is usually an extendable 8 foot long pool with a locking mechanism. The pole will extend to16 fee t depending upon the brand and style. The pole is used in many functions of pool service including netting, brushing and vacuuming the pool. I prefer the Elipto-lock brand pole as it can be extended and locked into place with a single twist of the pole and is very durable. There are many types of poles from aluminum to fiberglass of varying qualities. Check with your service manager to see which poles are available to you. Safety Tip! Always make sure that your pole and other equipment in the back of your service vehicle are safely secured to avoid load shift and losing any equipment while driving. Net: The net or also commonly called a “leaf rake” is a very important tool in pool service. It is attached to the pole and used to collect larger debris such as leaves, sticks and any other floating material on the water’s surface as well as collecting debris from the pool floor. There is a wide variety of brands and styles of new to be used. I prefer a lighter, more disposable net as they move faster in the water and are less expensive than the heavy duty nets. Some nets have replacement parts, while others are cheap and disposable. Fine mesh net: A fine mesh net is also a form of net; however it is made from a very fine mesh material and is used to collect very fine debris from the water’s surface or the pool floor. It can collect sand, dirt and grit and is a handy tool to use if the pool has very little debris and does not warrant vacuuming. 3 Wall brush: Wall brushes come in a variety of sizes and are usually made with nylon bristles as to not scratch the surface of the pool. A standard wall brush for use in pool service would be 18 inches however there are many larger and smaller sizes available. The wall brush attaches to the pole and is used to brush the sides of the pool surface, wiping away any dirt and debris. Steel wire wall brush: A steel wire wall brush is also a wall brush and comes in a variety of sizes. Its bristles are made of steel and are very sharp and abrasive. A steel wire wall brush should never be used on a fiberglass, painted, vinyl or other plastic lined type of surface as it can damage the surface. A steel wire brush attaches to the pole and is used to brush away and score or scratch the surface of algae to expose it to chemicals. Vacuum head: The vacuum head is a plastic, underwater vacuum device on rollers that attaches to your pole and to a vacuum hose. The vacuum head is rolled over the pool’s surface using the pole to direct it. I personally use an Excalibur vacuum head as I have found it to be sturdy, user friendly and long lasting however there are many available. Even if brand new, out of the box be sure that your vacuum head’s rollers are screwed in tight as the wheels do tend to unscrew and fall off from time to time. Brush-Vac vacuum head: A brush-vac is a vacuum head made with bristles, much like a wall brush and is made for vacuuming new plaster. New plaster should be brushvaced for the first 5 weeks as to not leave permanent roller marks from a regular vacuum on the soft new plaster surface. Brush-Vac Notes A Brush-Vac is only used on new plaster and in most cases will not be required to keep on your vehicle on a regular basis. Never use a regular vacuum on new plaster as it will leave permanent roller marks on the Vacuum head swivel: A vacuum head swivel is an new and soft plaster. Brush-Vac the accessory that attaches to the vacuum head and the hose plaster for the first 5 weeks. and causes the hose to spin freely allowing it better movement and preventing it from getting tangled and twisted up in the pool as you vacuum. Vacuum hose: The vacuum hose is the hose that connects to the vacuum head and directs the suction from the pools skimmer or suction line to the vacuum. With this suction the vacuum head can be rolled over the pool’s floor and debris is sucked up and sent into the filter where it is held and collected allowing the clean water to return to the pool. There are many sizes, styles and colors of vacuum hoses to choose from. I prefer the 50 foot Smooth Bore brand of hose as the inside of the hose is smooth, allowing water and debris to pass more quickly through it and the length of 50 feet is ample length to service any but the largest residential pools. The vacuum hose connects to the vacuum head. Vacuum leaf canister: A vacuum leaf canister is an attachment that the vacuum hose fits onto. It is a basket, enclosed in a clear plastic canister that collects debris vacuumed up before it reaches the pools pump basket or filter. Some pump baskets are very small and hold very little debris before the basket is filled or even breaks. The vacuum leaf canister holds a much larger amount of debris and is easily cleaned out. It is a very handy tool to have for large debris pools or during the wind season. Tile brush and tile soap: A tile brush is a short pole and replaceable brush or pad that is used to reach the tile of the pool. There are a couple different styles of tile brush but they are all similar and serve the same function. Brushing the tile cleans the scum or water line of the tile from debris and oils from swimmer waste, sun scream or any other impurities that might be sticking to the tile. 4 Brushing the tile does not remove calcium or staining. There are many tile soaps to use, all of them serving the same basic function. A small amount of the tile soap is applied to the tile brush pad and is quickly brushed around the tile of the pool. Bottled soap (Skimmer in a Bottle): Some technicians have been known to carry a bottle of “Skimmer in a Bottle”, “Liquid Skimmer”, “Naked Pool” or simply a bottle of dish washing liquid. A squirt of these soap based products across the center of a swimming pool will cause the surface debris to move to the sides of the pool making it easier to net out. This is an especially popular technique to use during the wind season when the days are shorter and there is much more debris in the pool. There are a few products available that do this however they are all soap based. The soap itself does not last long and does not affect the water chemistry. Chemical test kit: There are many chemical test kits available in the market. The most commonly used test kits use liquids to be mixed to gain chemical readings. There are also test strips that are dipped into the water to gain the same chemical readings. There is also a new electronic test device that is either dipped or held in the water or has its own test strips inserted into it to gain a reading. All of these test kits will need replacement items such as test reagents or liquids or test strips. A good test kit will test for the following: Did you know? Charlie Taylor is the pioneer and forefather of modern water chemistry as we know it today. He is the inventor of the “Taylor Test Kit”. Free and Available Chlorine (FAC) pH Balance Total Alkalinity (T.A.) Conditioner, Stabilizer (Cyanuric Acid) Calcium Hardness There are also test kits to test for Phosphates and Salt or Salinity levels that are handy to have on hand. Personally, I recommend and use the “Taylor Test Kit, Complete”. 1 lbs. D.E. scoop: It is always good to have a few 1 lbs. D.E. scoops on hand. A 1 lbs. D.E. (Diatomaceous Earth) scoop measures 1 lbs. of D.E., but can also contain up to about 3 lbs. of Dichlor or Trichlor chlorine, and other dry chemicals. They are useful in containing water should a surface need to be rinsed off. A 1 lbs. D.E. scoop will usually be orange. You will be able to tell how many lbs. of D.E. a scoop can hold by reading the description on the bottom of the cup. Hose coupler: a hose coupler is usually used to connect 2 vacuum hoses together. It is smooth, not barbed and is meant for under water use. A hose coupler is a handy tool for use when vacuuming a pool that has a skimmer with smaller than usually plumbing. Some skimmers have suction side plumbing that is too small to accommodate a hose and a hose couple acts as a reducer to help fit the hose into the skimmer. A hose coupler can be the difference between a difficult and slow suction vacuum and a fast vacuum. Tennis ball: Every now and again you will come across a pool that has two skimmers. When you plug your vacuum hose into one skimmer, due to water following the path of least resistance the suction is diverted over to the other skimmer causing you to lose most of your suction. You can plug a tennis ball into the other skimmer suction side hole forcing the suction to your vacuum. You never know when a tennis ball might be handy so it is good to always have one on hand. Never put an item into a skimmer that could get stuck in the plumbing. A tennis ball is large enough to plug the hole without risk of getting stuck inside the skimmer. 5 Spare bucket and rags: the swimming pool service business can often be messy with so many chemicals to use and cleaning to do. Having an extra bucket to hold chemicals, D.E. powder or other miscellaneous items that might be bouncing around in the bed of your truck can be very useful. Having extra rags to clean your hands, equipment or spills can also be handy. It’s always best to be prepared. Flash light and first aid kit: Because you never know. Miscellaneous tools: You’ll need a variety of tools for repairs and to take apart filters to clean them. A garden hose is also handy to have to clean filters. 2. Chemicals Found on a Typical Pool Service Vehicle Chlorine: 3 to 6 cases of liquid chlorine, 25 to 50 lbs. of Dichlor granular chlorine and/or 25-50 lbs. of Trichlor granular chlorine, 25 to 50 lbs. of 3 inch Trichlor tablets can all be commonly found on a service technician’s vehicle. Remember! Acid: 3 to 5 cases of Muriatic Acid Conditioner: Also called stabilizer or Cyanuric Acid, 20 to 100 lbs. of granular or powdered conditioner Each chemical has its own properties such as pH Balance. Get to know the chemical properties, proper handling, proper storage, handling and application of each of the chemicals in your arsenal. Each chemical will be covered in this manual. Algaecides: While there are many types of algaecides and such are covered in a later chapter, I personally carry 25 lbs. of Sodium Bromine (98%+ active ingredient) in a fine granular, quick dissolving form. YellowTrine is an excellent brand. I also commonly carry 5 to10 lbs. of Potassium Peroxymonopersulphate as an oxidizing assistant and water clarifier. As a phosphate removal agent I carry 3 to 6 liters of Phos-Free, however there are many different brands of phosphate removers that can be used. Metal based algaecides can also provide a good sanitizer provided they are well chelated. There are many forms of metal based algaecides. The PoolRX and Nature2 cartridges are examples of slow release metals while a bottle of Swimtrine or Silvertrine are both examples of liquid metal based algaecides. Diatomaceous Earth: D.E., or Diatomaceous Earth, also called filter powder is used for D.E. style filters to coat the filter grids. It is the filter media that actually holds the dirt and debris. 25 to 50 lbs. of D.E. are commonly carried. Soda Ash: Soda Ash (Sodium Carbonate) is used to increase the water’s pH Balance. 5 to 10 lbs. of Soda Ash is good to have on hand. Sodium Bicarbonate: Also called Baking Soda, Sodium Bicarbonate is used to increase the water’s Total Alkalinity. 5 to 10 lbs. of Sodium Bicarbonate is good to have on hand. 6 Things to Remember About Sections 1 and 2 There is a tool for every job. The more of these tools you get to know and master, the easier and more efficient your job will be. There is a different chemical for every condition. The more of these chemicals you get to know and master, the more efficient and knowledgeable of a technician you will be. Always be prepared with the right equipment and chemicals for the job. You never know what conditions you will come across from one service account to the next. 3. Three Keys to Clear, Clean and Healthy Water There are 3 keys to maintaining clear, clean and healthy swimming pool and spa water. These 3 keys are Circulation, Filtration, and Chemical Balance. Whenever your water becomes cloudy, unhealthy or algae begin to grow it can be due to a breakdown in one or more of these three key conditions. Circulation: Circulation is the flow or current in the water. The pump running daily for 6 to 8 or more hours per day for a pool, or 1 to 2 hours per day for a spa provides the water with circulation. Water circulation is important to keep the chemicals in the water evenly mixed, regulate the overall temperature of the water and make it more difficult for algae to settle and take root. When a body of water is not circulating it becomes stagnant. Circulation also helps get any sanitized and oxidized material in the water to the filter to be collected, keeping the water clear. Circulation Note Algae can grow even in very turbulent water as we can see in any stream. Running the pool pump for the appropriate amount of time each day will help to make it more difficult for the algae spores to settle however, circulation alone will not stop algae growth so do not believe that merely running the pump for a longer amount of time will resolve your algae problem. The algae starting in the grout in a pool skimmer is proof of this. Filtration: Filtration Note Filtration is the primary function of filtration. Water becomes cloudy when there is too much organic material in the water. Proper filtering will keep the water clear. Keeping your D.E. filters backwashed regularly will help the filter to operate more efficiently. Filtration is when the water is pushed through the pools filter. The filter collects any dirt and debris that the pump pushes into it or is vacuumed up, sending the clear and filtered water back into the pool. There are three standard types of filters that you will come across in swimming pool and spa service. These three filters are Diatomaceous Earth filters which have a series of grids inside them that hold a fine 7 powder filter media, Cartridge filters which have a large cartridge or a variety of smaller cartridges inside which collect the dirt and debris and Sand filters which are filled with a fine sand in a sectioned compartment and force the water through the sand with the sand collecting the dirt and debris. All of these types of filters should be cleaned and maintained regularly. The entire body of water should be passed through the filter at least once per day which is another reason to have the water circulate for 8 or more hours per day. Did you know? Chemical Balance: Chemical balance is the most important key to maintaining clean, clear and healthy water. Bad water chemistry can not only cause staining and damage to the pool’s surface and equipment but can also cause swimmer discomfort and illness. Proper water chemistry must be checked for and maintained each week. Unbalanced chemistry can allow for algae growth and algae harbors and protects germs and bacteria, allowing it to thrive and putting your swimmers health at risk. Chemical balance is the single most common reason for algae growth. Think of algae like a blood disease. When you see a symptom on the surface, merely brushing away the symptom does not cure the disease. The water must be treated in order to kill the algae else it will quickly return. There are 5 main aspects of water chemistry to keep balances and to check for regularly. These 5 aspects are: Free and Available Chlorine (FAC) - maintained at 3 to 5 ppm (parts per million) pH Balance (Power of Hydrogen) - maintained at 7.4 to 7.6 Total Alkalinity (T.A.) - maintained at 80 to 120 ppm (parts per million) Conditioner (Cyanuric Acid) - also called stabilizer, maintained at 70 to 90 ppm (parts per million) Calcium Hardness – maintained at 180 to 500 ppm (parts per million) There are a few other conditions that can also be checked for: Salt, Salinity (Sodium Chloride) - Salt is used with Salt generated chlorination systems only and should be maintained at 3250 to 3500 ppm (parts per million) though different manufacturers will have some variance in their recommended salinity levels Total Dissolved Solids (TDS) – a measurement of all solids dissolved in a sample of water and a general indication of the waters age. Proper TDS levels and recommendations will be given on a TDS test kit. Phosphates – a measurement of microscopic dead plant material and food for algae. Measurements for recommended phosphate levels will be given on any phosphate test kit. 8 Things to Remember About Section 3 All three keys, circulation, filtration and chemical balance are all important to maintain clear, clean and healthy water. Circulation is an important key as it helps to circulate the chemicals, it makes it more difficult for algae spores to settle and it helps sanitized and oxidized debris get into the filter. Filtration is the key keeping the water clear from oxidized organic material. Water becomes cloudy and loses its lustrous sparkle when too much organic material blocks the Sun’s rays from penetrating the water. Chemical balance is the main key to sanitizing and oxidizing the water and to preventing algae growth. 4. About Chlorine, Sanitizers and Oxidizers “Chlorine (IPA: /ˈklɔriːn/, from the Greek word 'χλωρóς' (khlôros, meaning 'pale green'), is the chemical element with atomic number 17 and symbol Cl. It is a halogen, found in the periodic table in group 17 (formerly VII, VIIa, or VIIb). As the chloride ion, which is part of common salt and other compounds, it is abundant in nature and necessary to most forms of life, including humans. In its common elemental form (Cl2 or "dichlorine") under standard conditions, it is a pale green gas about 2.5 times as dense as air. It has a disagreeable, suffocating odor that is detectable in concentrations as low as 1 ppm, and is choking and poisonous. Chlorine is a powerful oxidant and is used in bleaching and disinfectants. As a common disinfectant, Did you know? chlorine compounds are used in swimming pools to keep Should redness of the eyes occur from them clean and sanitary. In the upper atmosphere, chloramines a little known remedy is to chlorine-containing molecules have been implicated in rinse your eyes out with milk, rather the destruction of the ozone layer.” - Wikapedia Wow! That’s very fascinating information about chlorine from Wikapedia but what does that mean to the average swimming pool technician? Chlorine is a sanitizer that kills germs and bacteria and is also an oxidizer, much like a chemical fire, that burns up organic material including germs, bacteria, human waste such as sweat, dead skins cells, tears, mucous, urine and feces as well as other harmful organisms such as algae. than water. The reason for this is that milk is safe for human use and will neutralize and absorb the discomfort more rapidly, while water will only rinse and dilute it. This is the same principle as drinking milk, rather than water after eating spicy foods, to neutralize the burning sensation. When chlorine bonds with organic material it forms what is called a Chloramine. Chloramines are what cause that foul smell often associated with chlorine in a pool. When a swimmer has organic material such as dirt, dead skin cells, sweat, tears or mucous on them when they enter chlorinated water the chlorine will immediate bond with this material directly on the swimmer causing the all too familiar redness of the eyes and itchy or rashed skin. Simply rinsing off before entering the 9 pool or spa will remove most of this organic material making the water much more comfortable for swimmers. Chlorine is both an oxidizer and a sanitizer. A sanitizer is the chemical or device that kills or inactivates microorganisms present in pool and spa water. Microorganisms are too small to be seen with the naked eye and can only be observed through a microscope. This means that chlorine both kills or inactivates microorganisms and also burns away organic material. The Causes of Chlorine Demand: Did you know? Just one adult swimming vigorously for one hour will excrete over one pint of sweat? Gross, huh? That isn’t even mentioning the dead skin cells being rubbed off in the water from microfriction. Trust me on this. You really want to make sure that you always have plenty of sanitizer and oxidizer in your water. “Microorganisms are living creatures too small to be seen with the naked eye and are constantly introduced into the pool by rain, wind, and the human bather. Algae, bacteria, fungi, protozoans, yeasts and viruses are the kinds of organisms of concern. Most organisms are harmless to the human body but others are disease and infection causing. If not killed, these “germs” are transmitted via water to other swimmers. Non-living organic contaminants are also objectionalble. A study at Harvard University concluded that one active adult swimmer loses two pints of perspiration per hour. Perspiration is loaded with compounds resembling the chemistry of urine. The body is also constantly shedding microscopic skin particles sloughed off by the friction of water. These are all “involuntary wastes.” Add in “voluntary wastes” such as expectorate, nasal discharge, fecal matter and urine and you begin to appreciate the bather load created. Organics cause pool water to become dull, listless and cloudy. Periodic addition of an oxidizing chemical (called shocking, or superchlorinationg specifically when chlorine is used as the oxidizer) will rid the water of these contaminants, leaving it sparkling and inviting.” – Taylor Test Kit Pool & Spa Water Chemistry Guide In this section I will go over only the most common types of chlorine used for pool and spa chemical maintainance and their pros, cons and my opinions of them from field experience. These types of chlorine are as follows: Liquid Chlorine, Dichlor Granular Chlorine, Trichlor Granular Chlorine, 3 inch Trichlor Tablets, Calcium Hypochlorite and Salt. I will also go over Sodium Bromine and Potasium Monopersulfate as oxidizers, even though they are not categorized as chlorine. Liquid Chlorine: Liquid Chlorine is the most common and universally used form of chlorine in the swimming pool service industry. Liquid chlorine has some pros and cons you should be aware of before you decide to use it. It has a very high pH balance of about 13 so when you use it, for each gallon used you’ll have to add about 1 quart of Muriatic Acid to neutralize the high pH effects of the chlorine. Liquid chlorine is also heavy and consumes a large amount of space on your vehicle costing you fuel mileage and gas money. The more liquid chlorine you use, the more Muriatic Acid you will also have to keep on your vehicle to balance the chlorine’s high pH effects, consuming more space and adding more weight and less gas mileage to your vehicle. 10 Liquid chlorine is only chlorine. There is no additional conditioner or acid added to it as there are in other forms of chlorine. Liquid chlorine can be added directly to the pool water. Liquid chlorine is usually kept in white plastic bottles, preventing sunlight from deteriorating the chlorine before its use. Liquid chlorine will lose its effectiveness over time if not used, so it is recommended to not store it for long periods of time. Liquid chlorine is effective for use in fiberglass, vinyl lined, painted and tiled pools as they tend to be very sensitive to acid and will have a lower than normal pH balance. If the pH balance in a pool should become too low liquid chlorine is handy to use to help raise the pH balance. Did you know? The term “shock” as a marketing term is used to describe numerous different chemicals including Dichlor granular, Trichlor granular, Calcium Hypochlorite and even Potassium Monpersulphate. The chemical term for shock really means to “super chlorinate” or to bring to “break point” chlorination which is to raise the chlorine level to 10 ppm of free and available chlorine. “Shocking” the water is usually reserved for only the most extreme cases, however as a marketing term it is used quite liberally. As a technician it is good to know the difference between “shock” and to “super chlorinate”. Liquid Chlorine Note Always remember to dunk the bottom of your chlorine bottle into the pool water before and after every use to rinse any unseen chlorine drips and prevent them from leaving rings and possibly stains on the customer’s deck. One gallon of liquid chlorine will raise the free and available chlorine (FAC) of an average sized 20,000 gallon pool about 2 to 2.5 ppm (parts per million) depending upon the amount of organic material in the water, however this is only a rough estimation and many other factors will affect this outcome. Dichlor Granular Chlorine: Dichlor granular chlorine is an excellent form of chlorine, possibly the best to use as a service technician. The “Di” in Dichlor means two, meaning it has two things in it, both chlorine and conditioner, or stabilizer. The amount of conditioner in Dichlor granular is quite minimal but it does help maintain the conditioner level over long term use. 1 lbs. of Dichlor granular is about equivalent to 1 gallon of liquid chlorine making it far lighter and more compact and possible to carry large quantities of it on your service vehicle safely and in the case of an accident it will not spill and mix as liquid chlorine would. Dichlor granular chlorine is also in a fine granular form, quick dissolving and can be added directly to the water on any pool surface. Dichlor granular chlorine is pH neutral so no additional acid is required to neutralize it when added. Dichlor granular chlorine is sometimes referred to as “shock”, however this is just a marketing term and has little true meaning. Trichlor Granular Chlorine: Trichlor granular chlorine is a very good form of chlorine but can be limited in its usage. The “Tri” in Trichlor granular means three, meaning it has chlorine, conditioner and acid in it. The amount of conditioner in Trichlor granular is quite minimal but it does help maintain the conditioner level over long term use. The acid in Trichlor granular can be quite strong and is often used to remove algae from the surface of a white bottom pool as the acid in the Trichlor granular will dissolve away the algae. While it is often sold as an “algaecide”, one popular brand being called “Algae Ban” it is not formally an algaecide and is only a sanitizer and oxidizer like Dichlor but with a low pH. Trichlor granular is not as fine or quick dissolving as Dichlor granular and is intended to sit on the surface of the pool for a longer period of time. How long Trichlor granular takes to dissolve depends upon the water temperature and circulation of the water. If it is vacuumed up it will 11 dissolve very quickly, within a matter of minutes under the pressure of the filter. In colder temperatures Trichlor granular can take a long time, hours to dissolve. As a means of regular sanitation and oxidation, Trichlor granular can be added through the skimmer when the pump is on. Trichlor granular must NEVER BE USED on a dark bottom surface such as gray plaster else the acid in the Trichlor will cause staining to the surface, just as a dry granular form of acid would. Be careful when using Trichlor granular in a fiberglass, vinyl lined or other pH sensitive pool as the acid in the Trichlor may lower the pH balance of the pools water more than intended. If carefully used, Trichlor granular can be a very effective sanitizer and oxidizer to use in your pool water. I do not recommend it for use in above ground spas as it will stain and bleach the fiberglass surface and will affect the pH balance too severely. 1 lbs. of Trichlor granular is about equivalent to 1 lbs. of Dichlor granular or 1 gallon of liquid chlorine making it light weight and safe to transport compared to liquid chlorine. Be sure to always keep your Trichlor granular dry as any moisture will cause it to break down, releasing the chlorine and acid from the dry compound and cause mustard gas which is VERY hazardous to breathe in. Should any moisture, rain water for example, get into your container of Trichlor granular be sure and take a step back and allow the container to air out before retrieving the granules. 3 Inch Trichlor Tablets: 3 inch Trichlor tablets are the exact same chemical as Trichlor granular (See Trichlor granular on page 11) and should be handled with the same restraint and caution. 3 inch Trichlor tablets are made to dissolve slowly within a chlorinating device such as a 3 inch tablet floater or a plumbed in 3 inch tablet dispenser. 3 inch Trichlor tablets dissolve at a speed depending upon water temperature and circulation. In a floater in cold water 3 inch Trichlor tablets could take 2 to 3 weeks to dissolve. During the summer months in warmer water 3 inch Trichlor tablets could dissolve within a week. Trichlor tablets will dissolve much more quickly in a plumbed in tablet dispenser, also depending upon the water temperature but usually twice as fast as in a floater due to the pressure and circulation within the dispenser. 3 inch Trichlor tablets are not generally used as a primary form of chlorine but are more of a supplementary form of chlorine, helping to slow release more chlorine over the week between service visits and usually at times when the swimming pool is in heavier use. 3 inch Trichlor tablets must always be in a dispenser and are never allowed to sit on the pool’s surface as the acid within the Trichlor tablet will be very corrosive to the bottom of the pool, causing etching and other damage. 3 Inch Trichlor Tablet Note NEVER leave 3 inch Trichlor tablets in the skimmer basket. When the pump is not running the acid in the Trichlor tablets will dissolve and concentrate, flowing down the plumbing and into sensitive heater parts, pump parts and other places that a concentrated acid can do a lot of damage. Three 3 inch Trichlor tablets are roughly equivalent to 1 gallon of liquid chlorine, 1 lbs. of Dichlor granular or 1 lbs. of Trichlor granular however with it being so slow to dissolve it is best used as a supplementary form of chlorine. Always tie your floating 3 inch Trichlor tablet dispenser to a ring, hook or anything else you can find as to not let the floater float around active swimmers or to sit over the top step allowing the acid to slowly deteriorate the surface of the step. 12 Calcium Hypochlorite: Calcium Hypochlorite is often sold in 1 lbs. bags as a “shock” treatment. Commercially it can be bought in much larger quantities and is in a powdery granular form. Calcium Hypochlorite contains both calcium, which you do not want to increase in your water and also concentrated Did you know? chlorine. While Calcium Hypochlorite can produce pound for Calcium Hypochlorite is pound more oxidizing power than Dichlor granular or Trichlor also known as “dirty granular the amount of calcium in it is substantial and will raise chlorine” in the swimming the calcium hardness of your water fairly quickly if used with any pool industry due to its very regularity. Prematurely raising your calcium hardness will cause high calcium content and for many other water chemistry problems (see the chapter on the cloudy water effect it has calcium hardness) and age your water more quickly. Calcium Hypochlorite is in a quick dissolve granular form that can be for about 10 to 15 minutes added directly to the water on any surface of pool. DO NOT after adding it to the allow Calcium Hypochlorite to get wet while in its container or swimming pool water. when not in use. It is a powerful oxidizer and there is a substantial risk, if wet that it can increase in temperature and catch fire. Diluted and dissolved in the pools water, Calcium Hypochlorite is perfectly safe. 1 lbs. of Calcium Hypochlorite is about the equivalent of 1 ¼ gallons of liquid chlorine, 1 ¼ lbs. of Dichlor granular chlorine or 1 ¼ lbs. of Trichlor granular chlorine. This makes it very light weight to transport large quantities of in your vehicle. Calcium Hypochlorite has a pH Balance of 11 and may raise the waters pH Balance slightly, depending on how much is used. Calcium Hypochlorite does not contain any conditioner. While pound for pound Calcium Hypochlorite is the most powerful form of granular chlorine, due to its hazardous nature and negative side effects I recommend only using it sparingly or for extreme cases of algae as a super chlorination treatment. Salt (Sodium Chloride): Chlorine in its natural form is a gas and one of its most abundant sources is in salt, or Sodium Chloride. The chlorine gas is suspended within the salt crystal and when the Sodium Chloride is broken down it releases the chlorine gas from the crystal. Based on this premise salt is used to provide chlorine in salt generated chlorination systems. These salt chlorination systems have become increasingly popular as they have some very good sales points for the consumer. The salt water in the swimming pool, which is usually maintained at about 3250 to 3500 ppm (parts per million) Did you know? is very soft water and comfortable for the swimmer. The chlorine Salt (Sodium Chloride) produced by these systems is blended into the heavier salt is not only used to create solution in the water making it much less likely to detect any chlorine smell or harsh chlorine side effects. The salt in the pool chlorine with salt water is long lasting and once a level of 3250 to 3500 ppm is chlorination systems but reached only perhaps one 50 lbs. bag of salt per month, that chlorine, mainly depending on the size of the pool is all that is required, if even that from salt is also an to maintain the salt level, but always be sure to test the salinity important part of the before adding salt to get an accurate reading and to know exactly human body’s chemistry. how much salt to add. What the salesmen wont tell the customer is that salt generated chlorination systems produce a VERY high pH chlorine, about a pH of 13 actually and with this ever increasing pH condition much more Muriatic Acid must be added weekly. The system operates and produces chlorine for as long as the pool pump is operating, 8+ hours per day creating a continuously raising pH condition. To 13 balance and keep the water maintained at a pH balance of 7.4 to 7.6 and keep the chlorine effectively in its killing form almost double the regular amount of acid is required adding substantial additional costs for chemical maintenance as well as a constant pH bounce and a much more unstable Total Alkalinity. Another drawback of the salt generated chlorination system is that it has sensitive and expensive computer controls and a salt cell which is outside by the swimming pool equipment, exposed to temperature and the elements. The salt cell has to be inspected and cleaned once each 90 days. (See more information in the Salt Chlorination Systems chapter) An average sized 20,000 gallon pool would need about 400 lbs. of salt to start up (See the salt to water conversion chart the system and that is a lot of additional TDS (Total Dissolved Solids) to add to the water which will shorten the length of time until the pool should be drained and refilled and gives an extra risk of staining to the surface. While salt itself is very inexpensive the additional acid needed to balance the pH and the maintenance and repairs of the system itself can be very costly. Salt is also corrosive to some metals and should ONLY be used with a fiberglass or other plastic type of filter. Other Commonly Used Sanitizers and Oxidizers: Sodium Bromine: Sodium Bromine as a sanitizer and oxidizer works very similarly to chlorine, however it is not protected by conditioner as chlorine is and will therefore not last long in direct sunlight. Sodium Bromine is most commonly used in indoor pools, covered spas and as an algaecide. One very unique characteristic of Sodium Bromine is that a small amount of it will convert much of the Did you know? chlorine currently in the water into Sodium Bromine. In How Sodium Bromide works is that as an average sized 20,000 gallon pool with 3 to 5 ppm the Sodium Bromide (being a gas just like (parts per million) of free and available chlorine (FAC) it chlorine) is burned off, the chlorine in the water will sacrifice itself to “recharge” the only takes about 12 ounces of Sodium Bromine (98% active ingredient) to convert all of the chlorine in the Sodium Bromide. This is why when you pool into Sodium Bromine. Once the chlorine is test for chlorine after adding Sodium converted into Sodium Bromine it is consumed and Bromide or a Sodium Bromide based gone. When you test for chlorine the following week algaecide the chlorine will be depleted. there will be none. Sometimes, in the case of algae growth it is necessary to change your oxidizers as algae may build up a tolerance to one but will have never experienced another. (See the chapter about algae for more information about algae growth, tolerance and immunities) The following week when you service the pool be sure to add more sanitizer and oxidizer as the Sodium Bromine will not last unless the pool or spa is covered or protected from sunlight. Sodium Bromine is usually sold in a fine granular form but can also be found in a liquid form. The important thing to know when purchasing Sodium Bromine is to know the % of active ingredient. You want the Sodium Bromine to be at least 98% active ingredient when buying it in the granular form. Some products will want you to add their algaecide Sodium Bromine product weekly, which if done properly can be an effect and proactive way of preventing algae growth. Sodium Bromine can also be found in a slow dissolving 1 inch tablet form for use in covered spas and should only 14 be dispersed from within a 1 inch tablet floater. A good granular quick dissolve brand of Sodium Bromine is called “Yellow Trine” though there are many varieties of Sodium Bromine based algaecides available. Potassium Peroxymonopersulfate: Potassium is a non-chlorine based oxidizer often used as a “shock” treatment and water clarifier. Potassium comes in a very fine granular form and is often sold as a “shock” treatment in 1 lbs. bags or larger buckets. Potassium can be added directly to the water and can be used on any pool surface. While Potassium will oxidize, burning away any non-microbial organic contaminants and destroying any existing chloramines, it is not a sanitizer as chlorine or bromine are. Its primary function is to burn away contaminants and free up the chlorine to sanitize the water. The initial amount of Potassium to use for a pool is 2 lbs. per 10,000 gallons of water. After the initial use of Potassium to help keep the water clear and free up the chlorine from regular swimming pool use, use 1 lbs. per 10,000 gallons of water. Potassium contains no chlorine and won’t produce chloramines or chlorine related odors. It is safe to use on any surface and dissolves quickly. Potassium Monopersulfate should be used sparingly and is recommended to clarify heavily used pools and to free up and assist chlorine to sanitize the water. Additional Notes About Chlorine, Sanitizers and Oxidizers: pH Balance determines how much of the chlorine is in its “killing” form. The lower the pH Balance the more aggressive and effective the chlorine will be. It is recommended to keep the waters pH Balance at 7.4 to 7.6 ppm (parts per million). The higher your pH Balance is maintained the more likely it is that algae will build up a tolerance and be allowed to bloom and colonize as the chlorine is not effective enough to oxidize it before it builds up a tolerance and immunity. Swimmers should rinse off, at least their face with water before entering the chemically treated pool or spa water. Rinsing off will remove most surface organic material such as sweat, dead skin cells, dirt, tears and other swimmer waste which is what chlorine will combine with, creating chloramines and swimmer discomfort such as redness of the eyes, dry itchy skin and that foul smell associated with chlorine. Did you know? Potassium Peroxymonopersulfate was first developed by the DuPont Company in the mid-1950’s as an oxidizer for use in commercial products and processes. It is sold as a raw material to formulators under the trade name Oxone. Chlorine is protected, or stabilized by Conditioner (Cyanuric Acid). The Sun’s ultra violet rays break down chlorine and without protection chlorine will only last 24 to 48 hours in sunlight. For maximum protection it is best to keep the Conditioner level at 70 to 90 ppm (parts per million) but never above that as anything higher increases the risk of Conditioner staining. When Conditioner falls out of circulation and stains it causes a light purple staining of varying degrees. At 110 to 120 ppm the staining looks like a light dust coat of purple and can, depending on how severe be brushed off 15 and vacuumed. At 130+ the staining can no longer be brushed, deepens in color and also will be present purple vertical lines down the walls that looks like as though they were drawn in with a purple crayon. The Conditioner staining will cover both the Scam Alert! surface and plastic parts of the pool such Many large chain stores, department stores, as floor cleaners, chlorine tablet floaters discount stores and even grocery stores sell and skimmer baskets. It is my opinion from swimming pool chemicals. They will usually field experience that cold temperatures, sell them at a “discount” price making them an such as a cold winter night is one of many appealing option for home owners, however do conditions that can most likely drop not fall for this scam. The active ingredients of Conditioner out of circulation. With a these chemicals are often only half of what they Conditioner level of 70 to 90 chlorine will should be. Always purchase at a swimming pool last 2 to 3 weeks in the winter time when wholesale supplier or an actual swimming pool there is less direct sunlight and little to no supply store to insure that you aren’t paying swimmer use. 10% less to get 50% less. Chlorine levels should be maintained at 3 to 5 ppm (parts per million) of Free and Available Chlorine (FAC) 16 Things to Remember About Section 4 Chlorine is both a sanitizer (meaning it kills germs and bacteria) and an oxidizer (meaning it burns up organic material). Chloramines are created when chlorine bonds with and starts the process of sanitizing and oxidizing germs, bacteria and organic material and is also the most common cause of swimmer discomforts such as red irritable eyes and dry itchy skin. Rinsing off with water before swimming can remove much of the dead skin cells, dirt and human waste debris that can cause chloramines and make for a more enjoyable swimming experience. Liquid Chlorine has a high pH balance of about 13 and 1 quart of Muriatic Acid should be added for each gallon of Liquid Chlorine added to compensate for the high pH. Remember that adding additional Muriatic Acid on a regular basis will also affect the water’s Total Alkalinity. Dichlor granular is a pH neutral product, is quick dissolve and is safe to use in any type of pool. It is commonly referred to as “shock” however it is only made up of chlorine and conditioner and unless the Free and Available chlorine level is brought up to “break point” chlorination levels of 10 ppm than there is no actually “shocking” going on as shock is, for the most part just a marketing term to sell more product to uneducated home owners. Trichlor, whether in the granular of 3 inch tablet form is made up of chlorine, conditioner and acid and should be kept dry at all times while in its container. If Trichlor gets wet within an enclosed container than the chlorine and acid will break down, mix and form “mustard gas” which can be VERY hazardous. Never put 3 inch Trichlor tablets in the skimmer basket as the concentrated acid will accumulate when the pump is off and the acid can cause damage to the equipment. Never apply Trichlor granular to the surface of a dark or otherwise colored surface pool as the acid in the Trichlor can cause staining. Calcium Hypochlorite, also called “dirty chlorine” in the industry, is often referred to as “shock” and contains a substantial amount of calcium. It should be used sparing, if at all, and only in extreme cases of algae. Any use on a regular basis of Calcium Hypochlorite will cause the Calcium Hardness to raise and cause numerous other problems. Salt is naturally corrosive to metals and a salt chlorination system should never be used with a metal filter. The chlorine produced from salt has a very high pH Balance of about 13 as it is merely gas chlorine trapped within a sodium crystal. Using salt with a salt chlorination system to produce your chlorine also creates a high acid demand to keep the pH Balance maintained. 17 Sodium Bromide is also a sanitizer and oxidizer, just like chlorine, however it is not protected by Conditioner and is susceptible to being burned off by the Sun’s harmful U.V. rays. Sodium Bromide is also not affected by pH Balance and is ideal for use in above ground covered spas and for indoor pools and spas. Chlorine will sacrifice itself to replenish sodium Bromide. This is why after using Sodium Bromide in a chlorine pool or spa the chlorine level is often depleted, depending on how much sodium Bromide was used. Potassium Monopersulfate is a super oxidizer, however it does not sanitize. It is also often referred to as “shock”. Potassium Monopersulphate will immediately oxidize and break apart any chloramines in the water and therefore free up some otherwise combined chlorine, allowing that chlorine to focus more on sanitizing. Potassium Monopersulfate is also commonly used as a clarifier. Potassium Monopersulfate is quick dissolve and safe to use in any pool or spa. pH Balance determines chlorines ability to sanitize or kill germs and bacteria as well as how well it can oxidize organic material. The higher water’s pH Balance is the less effective and less in its killing form the chlorine is. Water’s pH Balance should be maintained at 7.4 to 7.6 to keep chlorine in its optimal killing form without risking low pH conditions which could result in corrosive water. The water’s Free and Available Chlorine (FAC) level should be maintained at 3 to 5 ppm (parts per million) to insure an ample and ready supply of sanitizer and oxidizer to keep the water pure and healthy. Conditioner (Cyanuric Acid), also called stabilizer gives the chlorine in the water a protective coating, shielding it from the Sun’s harmful U.V. rays, similar to the way that sunscreen protects us from a Sun burn. Without Conditioner chlorine, being it is after all only a gas, is quickly burned off from the Sun’s U.V. rays. On a sunny day the chlorine can be burned off within 24 hours, leaving the pool water unprotected from germs, bacteria, organic material build up and algae growth. Maintain the water’s conditioner level at 70 to 90 ppm. Chemicals distributed from department stores, discount stores, grocery stores, home improvement stores and others, while often offering price discounts usually only have about half of the active ingredients compared to the same chemicals found at a swimming pool store or a swimming pool wholesale distributor. 18 5. pH Balance (Power of Hydrogen) “pH is the measure of the acidity or alkalinity of a solution. It is formally a measure of the activity of dissolved hydrogen ions (H+), but for very dilute solutions, the molarity (molar concentration) of H+ may be used as a substitute with little loss of accuracy. In solution, hydrogen ions occur as a number of cations including hydronium ions (H3O+). In pure water at 25 °C, the concentration of H+ equals the concentration of hydroxide ions (OH-). This is defined as “neutral” and corresponds to a pH level of 7.0. Solutions in which the concentration of H+ exceeds that of OH- have a pH value lower than 7.0 and are known as acids. Solutions in which OH- exceeds H+ have a pH value greater than 7.0 and are known as bases (or alkalis). Because pH is dependent on ionic activity, a property which cannot be measured easily or fully predicted theoretically, it is difficult to determine an accurate value for the pH of a solution. The pH reading of a solution is usually obtained by comparing unknown solutions to those of known pH, and there are several ways to do so.” – Wikapedia A Note About pH The concept of pH was first introduced by Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909. Sørensen suggested the notation “PH” for convenience, standing for “power of hydrogen”, using the negative logarithm of the concentration of hydrogen ions in solution. That’s quite the defenition for pH Balance from Wikapedia and if you can understand it than you are a more knowlegable pool technician than I. So what does all this mean to us technicians in the field? pH stands for, depending on what language you go by: Potens Hydrogen (Latin for Hydrogen Power) Power of Hydrogen Potential of Hydrogen Pondus Hydrogenii (also Latin) Pouvoir Hydrogène (French) Did you know? Human blood has a pH Balance of 7.34 to 7.45. This is why keeping a pH Balance of about 7.4 to 7.5 is ideal as it is the same as that of the human body and the swimmer will not notice a pH difference when in the water, making the water more comfortable. Chlorine is also more in its killing form in lower pH conditions. Let’s simplify pH Balance into something a little more user friendly. To put it simply pH Balance is a scale to measure if a liquid is acidic or basic (Alkaline). It is a number from 0, representing the strongest acid to 14, representing the strongest basic. 7 on the pH Balance scale is a perfect neutral being neither acidic nor basic. Every liquid has a pH Balance measurement. Coffee and beer for example have a pH of 5 making them mildly acidic. Vinegar is a stronger acidic on the pH scale with a balance of 3. Ocean water has a pH balance of 8 making it mildly alkaline or basic. Ammonia is very alkaline or basic with a pH 19 measurement of 12. What does all this pH stuff mean in the swimming pool business? There are a few reasons why maintaining swimming pool water at a pH Balance of 7.4 to 7.6 is important. 1. Being that human blood has a pH Balance of 7.34 to 7.45, keeping the swimming pool water at a pH Balance of 7.4 to 7.6 will make it very close to that of humans and the water more comfortable for swimmer use. 2. Chlorine is a more aggressive sanitizer and oxidizer, in its killing form in lower pH balanced water. At a pH Balance of 7.4 to 7.6 most of the chlorine will be in its killing form. At a pH balance of 7.8 to 8.0 very little of the chlorine in the water is in its killing form. This will allow for organic material, germs and bacteria to remain in the water and algae to grow, colonize and build a stronger resistance to chlorine. Low pH Balance, below 7.0, will cause the water to become acidic and corrosive, causing pitting of the surface, metals to dissolve and fall out of concentration, staining of the walls and swimmer irritation and discomfort. Muriatic Acid Note Muriatic Acid is used to reduce water’s pH Balance and Total Alkalinity. When using Muriatic Acid be VERY careful not to spill it High pH Balance will cause scaling water, plugged filters, on the deck. Always dunk the bottom reduced circulation, cloudy water, chlorine inefficiency and of your acid bottle in the water before swimmer irritation and discomfort. and after use to rinse away any pH Balance is lowered by adding Muriatic Acid to the water, unseen acid drips that can otherwise cause severe staining to the as well as the addition of other low pH chemicals. pH customer’s deck. Balance is raised by adding Soda Ash (Sodium Carbonate)to the water, as well as the addition of higher pH chemicals. Water will naturally rise to a pH Balance of 8.0 on its own at a speed governed by the Total Alkalinity. Muriatic Acid Myth There is a myth about Muriatic Acid that states that if you pour it around the pool than it will affect the pH Balance more than the Total Alkalinity and if you pour it in one spot in the deep end than it affects the Total Alkalinity more than the pH Balance. This is a long standing and insubstantial myth that is completely false. ph Balance and Total Alkalinity (See chapter on Total Alkalinity) are related in that when you add Muriatic Acid to the water it lowers both of them. Total Alkalinity can also increase or decrease the effectiveness of Muriatic Acids ability to lower the pH balance. If there is too much Alkaline material in the water it will absorb the acid too quickly, not allowing it to lower the pH Balance as much as intended. Things to Remember About Section 5 pH (Power of Hydrogen) is the measure of the acidity or alkalinity of a solution. pH Balance should be kept at 7.4 to 7.6 at all times. pH Balance determines chlorine’s ability to effectively sanitize and oxidize. Muriatic Acid is used to lower pH Balance. Soda Ash is used to raise pH Balance. pH balance below 7.0 is acidic and can cause pitting to the pool’s surface, metals to dissolve and fall out of circulation, staining on the surface and swimmer discomfort. A high pH Balance can cause scaling, 20 plugged filters, cloudy water, chlorine inefficiency and swimmer irritation. 6. Total Alkalinity (T.A.) “Alkalinity or AT is a measure of the ability of a solution to neutralize acids to the equivalence point of carbonate or bicarbonate. Alkalinity is closely related to the acid neutralizing capacity (ANC) of a solution and ANC is often incorrectly used to refer to alkalinity. The alkalinity is equal to the stoichiometric sum of the bases in solution. In the natural environment carbonate alkalinity tends to make up most of the total alkalinity due to the common occurrence and dissolution of carbonate rocks and presence of carbon dioxide in the atmosphere. Other common natural components that can contribute to alkalinity include borate, hydroxide, phosphate, silicate, nitrate, dissolved ammonia, the conjugate bases of some organic acids and sulfide. Solutions produced in a laboratory may contain a virtually limitless number of bases that contribute to alkalinity. Alkalinity is usually given in the unit mEq/L (milliequivalent per liter). Commercially, as in the pool industry, alkalinity might also be given in the unit ppm or parts per million. Alkalinity is sometimes incorrectly used interchangeably with basicity. For example, the pH of a solution can be lowered by the addition of CO2. This will reduce the basicity; however, the alkalinity will remain unchanged.” – Wikapedia Wow! There is another mouthful from Wikapedia. Let’s break that down into something we can apply to swimming pool water chemistry. Total Alkalinity is a measurement of alkaline particles in the water. These alkaline particles determine the ability of water to resist changes in the pH. Alkaline particles can neutralize acid acting as a “buffer”, protecting water from wide pH increase or decrease. Essentially, Sodium Bicarbonate (Baking Soda) dissolved in pool water, or what we call Total Alkalinity (T.A.) is the “buffer” that absorbs acid, therefore governing the pH and protects the pool’s surface and equipment from the effects of acid. Did you know? pH Balance and Total Alkalinity are related in that Muriatic Acid effects them both simultaneously. They are also related in that Total Alkalinity determines the speed in which pH Balance rises and falls. Always be mindful of both the pH Balance and the total alkalinity when checking the water’s chemistry as they are quite close relatives. Without alkaline particles in the water, the water will suffer from a drastic pH bounce meaning a rapid fluctuation of pH levels with the addition of small amounts of acid, base (alkali), or other pHaltering agents. The result is a highly unbalanced water condition resulting in damage to copper heat exchangers, light rings, stainless steel ladders, and concrete pool surfaces. 21 A Thing or Two (or Three) About Carbonates There are three dry chemicals that effectively raise pH Balance, Total Alkalinity and both at the same time. Sodium Carbonate, or commonly called Soda Ash is effective in raising just the pH Balance. This is handy to have around when you have added too much acid, particularly in a fiberglass pool. Sodium Bicarbonate, or commonly called Baking Soda is effective in raising just the Total Alkalinity. This is handy to have when you forgot to keep track of the Total Alkalinity and your pH balance is getting out of control. Sodium Sesqui-bicarbonate is an even mix of both Sodium Carbonate and Sodium Bicarbonate and is effective in raising both the pH Balance and the Total Alkalinity. All three dry powdery chemicals are safe to use in any pool and will cloud the water temporarily after use. Problems from a low Total Alkalinity include corrosive water, pitting of the surface, metals dissolve, staining of the walls and pH bounce. Problems from a high Total Alkalinity include scaling water, plugged filters, reduced circulation, cloudy water and an increased rise in pH balance. Total Alkalinity is lowered by adding Muriatic Acid or other low pH product to the swimming pool water. Total Alkalinity is raised by adding Sodium Bicarbonate (Baking Soda) to the swimming pool water. The ideal range to maintain Total Alkalinity is from 80 to 120 ppm (parts per million). Remember, when you add Muriatic Acid to the water it will lower both the Total Alkalinity and the pH Balance. While pH Balance will change rapidly, changing from one day to the next, Total Alkalinity rises and falls slowly, over weeks rather than days. As water is added to the swimming pool each week alkaline material is also added which will gradually raise the Total Alkalinity. 22 Things to Remember About Section 6 Total Alkalinity is a measurement of alkaline particles in the water. These alkaline particles determine the ability of water to resist changes in the pH. Sodium Bicarbonate (Baking Soda) dissolved in pool water, or what we call Total Alkalinity (T.A.) is the “buffer” that absorbs acid, therefore governing the pH and protects the pool’s surface and equipment from the effects of acid. The ideal range to maintain Total Alkalinity is from 80 to 120 ppm. Muriatic Acid is used to lower the water’s Total Alkalinity Sodium Bicarbonate (Baking Soda) is used to raise the water’s Total Alkalinity. Problems from a low Total Alkalinity include corrosive water, pitting of the surface, metals dissolve, staining of the walls and pH bounce. Problems from a high Total Alkalinity include scaling water, plugged filters, reduced circulation, cloudy water and an increased rise in pH balance. Muriatic Acid lowers both pH Balance and Total Alkalinity. 7. Conditioner, Stabilizer (Cyanuric Acid) Conditioner (Cyanuric Acid), also called Stabilizer protects chlorine from the Sun’s harmful Ultra Violet rays. The term “Stabilized Chlorine” means that there is also Conditioner in the chlorine such as Dichlor and Trichlor. Liquid Chlorine and Calcium Hypochlorite do not have any Conditioner added to them. Without Conditioner in the water chlorine will only last a short while, about 24 to 48 hours in direct sunlight. With the proper amount of Conditioner in the water chlorine can last about 1 to 3 weeks in the water, depending upon bather load and temperature and considering that chlorine is a very large expence in the swimming pool service business it is best to keep the chlorine in the water for as long as possible. Also, you want to have a very stable and continuous level of chlorine in the water to prevent chlorine bounce or a weakness in your sanitation and oxidation system that will allow germs and bacteria to thrive and algae to grow and colonize. Conditioner should always be maintained at 70 to 90 ppm (parts per million) but never above that due to the increased risk and severity of Conditioner staining on the pools surface. When Conditioner falls out of circulation and stains the pools surface it is clearly identified as a light purple staining of varying degrees. It will stain any surface as well as plastics and emtals in the water such as skimmer baskets, floor cleaners and light fixture rings. At 110 ppm Conditioner staining will look like a light dust coat of purple that can be brushed away and vacuumed up At 120 ppm Conditioner staining will look like a moderate dust coat of purple that can not be brushed up or vacuumed 23 At 130 or higher Conditioner staining will look like a deep dust coat or purple staining as well as purple verticle lines, as if drawn in with a crayon along the walls Conditioner Note Conditioner is acidic and if allowed to sit on the deck or if it sits at the bottom of the pool it can cause etching and staining. Always add it through the skimmer slowly and allow the pump to run for at least 4 hours to break it down properly. Conditioner is removed from the water by draining, backwashing, splash, bather carry out or overfill. Expect to lose from 10 to 30 ppm of Conditioner during the hot summer months from heavy usage. Conditioner levels below 70 ppm (parts per million) tend to be less effective and the chlorine in the water is burned off by the Sun’s harmful Ultra Violet rays more rapidly. This condition allows your chlorine level to bounce more drastically between visits causing a weakness in your oxidation and sanitation system and giving algae the opportunity to grow and colonize. While most other water chemistry instruction manuals recommend maintaining Conditioner levels from 30 to 70 I recommend keeping a level of 70 to 90, but not above. Conditioner is sold to the swimming pool industry in 3 different forms. The most commonly used form of Conditioner is in the slow dissolving granular form. Conditioner is also sold in a fine powdered form. The third and far less common form of Conditioner is sold in a liquid solution. The liquid solution is a fairly new product on the market and is drastically more expencive compared to its granular and powdered counterparts. The powdered form of Conditioner, pound for pound to my experience tends to be more concentrated by about 10% or more so be careful when applying it to the pool water to not add too much and to check the Conditioner level frequently. Conditioner is added through the skimmer slowly while the pump is running and the pump should be allowed to run for about 4 hours to allow the Conditioner to break down in the filter and be distributed into the pool water. Here are some notes to remember when adding Conditioner to the pool: Always add Conditioner through the skimmer while the pump is running. Allow the pump to run for about 4 hours after Conditioner is added to allow it to break down in the filter and be distributed into the water. Add the Conditioner slowly to the skimmer, only a cup or two at a time over a few minutes at a time to prevent the pump basket from getting clogged up with the Conditioner and stopping the pumps suction. Increase the Conditioner level slowly, not all at once to prevent over conditioning. Take a few weeks if need be. NEVER add Conditioner directly to the pool as Conditioner is an acid and if allowed to sit on the surface it will cause pitting, etching and staining to the surface. If you need to lower the Conditioner level of the water the pool can be drained with a submersible pump. Partially drain the pool by placing the pump on the top or second step if only a small amount of Conditioner needs to be removed. 24 Things to Remember About Section 7 Conditioner (Cyanuric Acid), also called Stabilizer protects chlorine from the Sun’s harmful Ultra Violet rays. The term “Stabilized Chlorine” means that there is also Conditioner in the chlorine such as Dichlor and Trichlor. Conditioner should always be maintained at 70 to 90 ppm (parts per million) but never above that due to the increased risk and severity of Conditioner staining on the pools surface. Always add Conditioner slowly through the skimmer while the pump is running. Allow the pump to run for about 4 hours after Conditioner is added to allow it to break down in the filter and be distributed into the water. Increase the Conditioner level slowly, not all at once to prevent over conditioning. Take a few weeks if need be. NEVER add Conditioner directly to the pool as Conditioner is an acid and if allowed to sit on the surface it will cause pitting, etching and staining to the surface. 8. Calcium Hardness “Hard water is the type of water that has high mineral content (in contrast with soft water). Hard water minerals primarily consist of calcium (Ca2+), and magnesium (Mg2+) metal cations, and sometimes other dissolved compounds such as bicarbonates and sulfates. Calcium usually enters the water as either calcium carbonate (CaCO3), in the form of limestone and chalk, or calcium sulfate (CaSO4), in the form of other mineral deposits. The predominant source of magnesium is dolomite (CaMg(CO3)2). Hard water is generally not harmful. The simplest way to determine the hardness of water is the lather/froth test: soap or toothpaste, when agitated, lathers easily in soft water but not in hard water. More exact measurements of hardness can be obtained through a wet titration. The total water 'hardness' (including both Ca2+ and Mg2+ ions) is read as parts per million or weight/volume (mg/L) of calcium carbonate (CaCO3) in the water. Although water hardness usually only measures the total concentrations of calcium and magnesium (the two most prevalent, divalent metal ions), iron, aluminium, and manganese may also be present at elevated levels in some geographical locations. Hardness in water is defined as the presence of multivalent cations. Hardness in water can cause water to form scales and a resistance to soap. It can also be defined as water that doesn’t produce lather with soap solutions, but produces white precipitate (scum). Effects on skin 25 Some confusion may arise after a first experience with soft water. Hard water does not lather well with soap and leaves a "less than clean" feeling. Soft water lathers better than hard water but leaves a "slippery feeling" on the skin after use with soap. For example, a certain water softener manufacturer contests that the "slippery feeling" after showering in soft water is due to "cleaner skin" and the absence of "friction-causing" soap scum. However, the chemical explanation is that softened water, due to its sodium content, has a much reduced ability to combine with the soap film on your body and therefore, it is much more difficult to rinse off. Solutions are to use less soap or a synthetic liquid body wash. Hard water in the US According to the United States Geological Survey, 89.3% of US homes have hard water. The softest waters occur in parts of the New England, South Atlantic-Gulf, Pacific Northwest, and Hawaii regions. Moderately hard waters are common in many of the rivers of the Tennessee, Great Lakes, Pacific Northwest, and Alaska regions. Hard and very hard waters are found in some of the streams in most of the regions throughout the country. Hardest waters (greater than 1,000 mg/L) are in streams in Texas, New Mexico, Kansas, Arizona, and southern California. “ – Wikapedia That was a gross overkill of information about hard water from Wikapedia, but still quite educational. So here we are again, wondering just what that means to us in the backyard by the swimming pool. Here is a breakdown of what Calcium Hardness means to us. Did you know? The term “hard” in relation to hard water comes from the laundry detergent industry and means that it is “hard” to form suds. In wash water the minerals combine with soap to form a grey insoluable curdlike scum, making cleaning less effective more expencive. Water that contains little or no Calcium or Magnesium is called soft. Water that contains high levels of Calcium and Magnesium salts is called hard. Magnesium does not form scale, so interest here is focused on Calcium. Pools having too much Calcium may scale, but pool water entirely deprived of Calcium becomes aggressive and seeks to dissolve Calcium into the water from contact surfaces, such as grouting, concrete and plaster. High Calcium Hardness can be reduced by partially or completely draining the pool and refilling with fresh water of lower hardness. Low Calciuim Hardness can result in corrosive water, etching of the plaster, pitting of concrete, dissolving of grout and pitting of pool decks and can be raised by adding a Calcium Elevator. High Calcium Harndess can result in scaling water, plugged filters, reduced circulation, cloudy water, heater inefficiency and additional calcium deposits on the tile and spill ways. Calcium Hadness should be maintained at 180 to 500 ppm (parts per million). The pool should be drained and refilled past 500 ppm. 26 Things to Remember About Section 8 Water that contains little or no Calcium or Magnesium is called soft. Water that contains high levels of Calcium and Magnesium salts is called hard. High Calcium Hardness can be reduced by partially or completely draining the pool and refilling with fresh water of lower hardness. Low Calciuim Hardness can result in corrosive water, etching of the plaster, pitting of concrete, dissolving of grout and pitting of pool decks and can be raised by adding a Calcium Elevator. High Calcium Harndess can result in scaling water, plugged filters, reduced circulation, cloudy water, heater inefficiency and additional calcium deposits on the tile and spill ways. Calcium Hadness should be maintained at 180 to 500 ppm (parts per million). The pool should be drained and refilled past 500 ppm. 9. Algae! Know Your Enemy “Algae are single-cell plants containing chlorophyll. They are some of the hardiest and most widespread organisms living on the planet, existing in over 30,000 different varieties. Algae require warm water, sunlight, and carbon dioxide to grow – and pool water has the potential of providing just such an enviornment.” – Taylor Test Kit Pool & Spa Water Chemistry Guide Algae are not only unsightly in the swimming pool but also harbor and protect germs and bacteria that can be harmful to swimmers. Algae is one of the top two reasons a customer will fire a swimming pool technician. The other reason is not showing up regularly to service the swimming pool. There are three main categories of algae found in pool water, Mustard (yellow) Algae, Green Algae and Black (blue-green) Algae. Each will be described in detail here. Mustard (yellow) Algae: “Yellow-green algae or xanthophytes are an important group of heterokont algae. Most live in freshwater, but some are found in marine and soil habitats. They vary from single-celled flagellates to simple colonial and filamentous forms. Unlike other heterokonts, their chloroplasts do not contain fucoxanthin, which accounts for their lighter colour. They appear to be the closest relatives of the brown algae.” – Wikapedia 27 Mustard (yellow) Algae appears as a yellow powdery deposit on the pool, usually on the shady side. Once established, it is resistant to chlorine and can exist in the presence of 3.0 to 5.0 ppm (parts per million) or Free and Available Chlorine (FAC). Mustard (yellow) Algae, if untreated can become very strong and will take additional time to remove from the walls and surface of the pool. Green Algae: Did you know? “The green algae (singular: green alga) are the large The first places that algae will usually start group of algae from which the embryophytes (higher to grow are on the grout inside the skimmer, plants) emerged. As such, they form a paraphyletic group, along the ring of the light or on the string although the group including both green algae and that may tie down a 3 inch chlorine tablet embryophytes is monophyletic (and often just known as floater. You can usually see the algae using kingdom Plantae). The green algae include unicellular and polarized and U.V. protected Sun glasses colonial flagellates, usually but not always with two before you can see it with the naked eye. flagella per cell, as well as various colonial, coccoid, and So now you know that those Sun glasses filamentous forms. In the Charales, the closest relatives of aren’t just for looking cool…but they help. higher plants, full differentiation of tissues occurs. There are about 6000 species of green algae. Many species live most of their lives as single cells, while other species form colonies or long filaments. A few other organisms rely on green algae to conduct photosynthesis for them. The chloroplasts in euglenids and chlorarachniophytes were acquired from ingested green algae, and in the latter retain a vestigial nucleus (nucleomorph). Some species of green algae, particularly of genera Trebouxia or Pseudotrebouxia (Trebouxiophyceae), can be found in symbiotic associations with fungi to form lichens. In general the fungal species that partner in lichens cannot live on their own, while the algal species is often found living in nature without the fungus.” – Wikapedia Green Algae is usually a floating algae, but sometimes clings to walls. Pool water becomes turbid with a green growth that renders the pool uninviting and dangerous to use by making it slippery and difficult to see the bottom of the pool. Before the green color appears, the walls of the pool surface feel slick, water becomes hazy and it exhibits a high chlorine demand. If untreated, Green Algae can grow and colonize rapidly, spreading over the entire pool and water surface. Black (blue-green) Algae: “Cyanobacteria, also known as blue-green algae, blue-green bacteria or Cyanophyta, is a phylum of bacteria that obtain their energy through photosynthesis. The name "cyanobacteria" comes from the color of the bacteria (Greek: κυανός (kyanós) = blue). They are a significant component of the marine nitrogen cycle and an important primary producer in many areas of the ocean, but are also found on land. Stromatolites of fossilized oxygen-producing cyanobacteria have been found from 2.8 billion years ago. The ability of cyanobacteria to perform oxygenic photosynthesis is thought to have converted the early reducing atmosphere into an oxidizing one, which dramatically changed the composition of life forms on Earth by provoking an explosion of biodiversity and leading to the near-extinction of oxygen-intolerant 28 Black Algae Warning! If one of your accounts develops Black Algae than you, my friend have quite a problem on your hands. Black Algae is the plague of all pools. It is the most difficult to treat form of algae that can grow in a swimming pool or spa. Let’s face it, Black Algae has been around for over 2.8 billion years. Black algae has a VERY tough protective outer shell that even a steel wire brush usually can’t scrape off and roots that grow so deep they reach into the gunite of the pool, causing the algae to return, usually in the same spots over and over again over the years, even after a drain and acid wash. organisms. Chloroplasts in plants and eukaryotic algae have evolved from cyanobacteria via endosymbiosis.” – Wikapedia More Black Algae Warnings! Really, no kidding around here, Black Algae really is that bad. If you use a wall brush of any kind, steel or otherwise on Black Algae be sure and rinse it off afterward with Liquid Chlorine, else there is a very real chance that you can transfer the Black Algae into other pools that you use those same brushes on. Scrubbing Black Algae with a steel wire brush really only scores or cuts the surface of it to allow the chemicals a chance to get past the Black Algae’s tough outer shell. Black (blue-green) Algae is evident by the formation of small dime (or smaller) to quarter sized black (or blue-green) spots, tenaciously adhering to the pool’s surfaces. These spots have a very slippery coating and are like thick nodules to the touch. Black (blue-green) Algae forms a layered structure where the first layers, which may be killed by chlorine, protect underlayers from further destruction. Black (blue-green) Algae, like Mustard (yellow) Algae, is also chlorine resistant. Black (blue-green) Algae can penetrate deep into the pool’s surface once established, making it very difficult to destroy and also likely to return in the presence of unbalanced water. Black (blue-green) Algae is the rarest form of algae you will encounter however is, by far the most tenacious and difficult to destroy. Algae Formation: When the sanitizer level is allowed to deplete (your chlorine level gets low), if your pH Balance becomes too high making the chlorine ineffective and unable to sanitize and oxidize or when the phosphate level (microscopic dead plant material and food for algae) is too high algae spores (seeds) will germinate. These Algae Note algae spores are found in the air, the fill water and all around The entire pool should be treated the swimming pool area and are constantly present in the for algae immediately when algae water, waiting for the opportunity to bloom. Within 12 hours, a is first seen Brushing it away will pool can be completely overrun with Green Algae. This only redistribure the algae that is condition is called Algae Bloom. Green Algae and Mustard seen but will not cure the problem (yellow) Algae blooms can be totally destroyed by and the following service visit the superchlorination to 30 ppm (parts per million) of Free and algae problem will be much worse Available Chlorine (FAC). Even after superchlorinating, once and the customer will not likely be the chlorine levels have returned to 3.0 to 5.0, if the water happy about it. becomes chemically unbalanced algae can return. There are other means of destroying algae and Algae Blooms without superchlorination (see the chapter on Algaecides and Treatments for Algae). 29 Things to Remember About Section 9 Algae are not only unsightly in the swimming pool but also harbor and protect germs and bacteria that can be harmful to swimmers. Algae is one of the top two reasons a customer will fire a swimming pool technician. Mustard (yellow) Algae appears as a yellow powdery deposit on the pool and once established, it is resistant to chlorine. Green Algae is usually a floating algae, but sometimes clings to walls. Pool water becomes turbid with a green growth that renders the pool uninviting and dangerous to use by making it slippery and difficult to see the bottom of the pool. “The green algae (often just known as kingdom Plantae) are the large group of algae from which the embryophytes (higher plants) emerged. There are about 6000 species of green algae. Many species live most of their lives as single cells, while other species form colonies or long filaments. Stromatolites of fossilized oxygen-producing cyanobacteria (blue-green algae) have been found from 2.8 billion years ago. Black (blue-green) Algae is evident by the formation of small dime (or smaller) to quarter sized black (or blue-green) spots, tenaciously adhering to the pool’s surfaces. Black (blue-green) Algae, like Mustard (yellow) Algae, is also chlorine resistant. Black (blue-green) Algae can penetrate deep into the pool’s surface once established, making it very difficult to destroy and also likely to return in the presence of unbalanced water. When the sanitizer level is allowed to deplete (your chlorine level gets low), if your pH Balance becomes too high making the chlorine ineffective and unable to sanitize and oxidize or when the phosphate level (microscopic dead plant material and food for algae) is too high algae spores (seeds) will germinate. 10. Algaecides and Phosphate Removers “Algaecides are chemicals added to pool water to control algae. While algaecides at high dosage can kill algae, most are utilized as algaestats, preventing algae fromation when chlorine is allowed to become depleted. Consider them insurance policies against sloppy chlorination. There are three main groups of algaecides: 1. Quats: Quaternary Ammonium Compounds (commonly referred to as “quats” and abbreviated to (QAC) are organic nitrogen substances that kill algae by disrupting the function of their cell membranes. Quats work best on Green Algae. They’re the least expensive and best-selling algaecides but tend to foam, especially in spas, when added in excess. 2. Polyquats: are non-foaming algaecides sold in concentrations of 30% to 60%, as compared to quats, which are usually between 5% to 10% active. While more costly than quats, polyquat 30 algaecides are very effective not only on Green Algae, but also with the chlorine-resistant Mustard and Black Algae. 3. Copper Salts: Copper Ion is a very effective algaecide and is used in ponds and lagoons as well as pools to kill and prevent algae formations. The downside to copper usage is stain formation. Over time, soluble copper salts will precipitate from pool water and deposit on pool walls, creating a bluing effect. Then, in the presence of chlorine, these salts will turn to copric oxide that causes greyto-black staining of the pool walls.” – Taylor Test Kit Pool & Spa Water Chemistry Guide This explanation of algaecides from the Taylor Test Kit Water Chemistry Guide is rather complex and not Always add your liquid metal based entirely helpful in the field when, as a technician you algaecides through the skimmer while the have a very large variety of algaecides to choose from. pump is running. If you add it directly to Simply asking the person at the counter of your local the pool you risk staining along the tile, swimming pool supply store to show you a selection of artificial rock or other surface material at Quat, Polyquat and Copper Salt algaecides will likely just the water line. Always read the get both him and you even more confused. Lets break instructions on the algaecide carefully down the mistique of algaecides into something we can before adding it to the water. use in the field. There are three kinds of algaecides I will go over here, even though there are others available in the market. To my experience, these three are the primary algaecides used in the Did you know? field; Metal based algaecides, Sodium Bromine and Phosphate In the early days of removers: Metal Based Algaecide Note swimming pool service they used to use colloidal gold as an algaecide. That must have cost more than a pretty penny. Metal Based Algaecides: There are many metal based algaecides in the market today. They are all very popular and commonly used. The three main metals used in these algaecides are Zinc, Copper and Silver. You can find these metals in brands such as SwimTrine (copper), SilverTrine (silver), Zinc Ball (zinc), (yes, its actually a large metal ball made of zinc that is placed in the skimmer or pump basket and breaks down over time) and Nature2 cartridges (cartridges plumbed directly into the pools plumbing that contain a combination of small metal granules that break down and distribute their metals into the water. The cartridges run out of metal over time and need to be replaced, usually over about 4 to 6 months depending on the size of the cartridge). There is also a product called the PoolRX that works the same as a Nature2 cartridge, however instead of small metal pellets or granuals the PoolRX uses a metal cyliner made up of Zinc, Copper and Silver and breaks down from the water’s circulation by placing it in the pump basket. The PoolRX cylinder also has to be recharged or replaced every 4 to 6 months. Metals are natures own sanitizers and can even increase the effective sanitation power of chlorine when used together, however metals do not oxidize the water. When a metal based algaecide or water purifier is used it is always best to also supplement with Potassium Monopersulfate or another form of oxidizer such as chlorine or Sodium Bromine. Zinc is the weakest of these oxidation increasers, copper is the most common and silver is the strongest currently sold in the market. The risk of using a metal based algaecide or water purifier is that abundant amounts of 31 metals in the water can fall out of circulation and cause staining over the pool’s surface. You want to be very careful to use a metal based algaecide or purifier that has a chelating (stain protection) agent in it as well. Liquid metal based algaecides are usually added through the skimmer while the pump is running. This prevents any film or residual from floating on the surface of the water and staining the tile or waterline and also gives a more even distribution into the water through the return lines. Always follow the application procedures on the label of such a product. Sodium Bromine Based Algaecides: Sodium Bromine is discussed at length in the “About Chlorine, Sanitizers and Oxidizers” chapter on page 14. Here we will discuss Sodium Bromine when used as an algaecide. There are a number of algaecide products in both granular and liquid form that use Sodium Bromine as their active ingredient. The liquid products tend to have far less active ingredientand more unlisted inert ingredients as to be easier to simply squirt across the surface of the water once or twice per week as a regular maintainance product. The granular forms of Sodium Bromide are often more concentrated at 88% to 98% however still suggest a regular weekly use of 1 to 2 ounces per week. Some of these Sodium Bromine based algaecides are YellowTreat (88%), YellowTrine (98%) and No-Mor Problems. Sodium Bromine, once broken down does not remain in the pool as metal based algaecides do and does not cause any staining to the pool surface. It is an sanitizer and oxidizer just as chlorine is but has a different chemical make-up. Should algae begin to grow and colonize despite balanced chemistry than that algae, having been born and bloomed with chlorine present will be very resistant. Algae is a living organism, just as humans are and has an immune system to protect it, just as humans do. Just as humans can get a flu shot, which a small dosage of the most common strain of flu viruse at the time to help the human immune system build up a resistance against it before a larger outbreak occurs, algae blooming when chlorine is present makes it quite resistant in a very similar way. When this tolerance to chlorine is created, simply changing your sanitizer and oxidizer to something chemically different that algae has not yet experienced can often be the most effective way to kill it. Sodium Bromine will naturally break down over time in the water, usually over the span of a week, and will also be further broken down by direct contact with the Sun’s harmful Ultra Violet rays. Chlorine in the water will sacrifice itself to recharge Sodium Bromine and often, after adding a Sodium Bromine based algaecide to the pool water you will find that the following week all of your Free and Available Chlorine (FAC) is gone or at a zero reading. It is adviced to buffer your chlorine, adding a bit more than usual before adding a Sodium Bromine based algaecide to help keep the Sodium Bromine fully charged and effective for as long as possible. 32 Sodium Bromine Myth The myth about Sodium Bromide is that it will consume all the chlorine in the water. This is a myth and is incorrect. Sodium Bromide is not protected from the Sun’s U.V. rays. When exposed to direct sunlight Sodium Bromine begins to break down. Chlorine sacrifices itself to “recharge” the Sodium Bromine. As long as there is chlorine in the water it will continuously sacrifice itself to keep the Sodium Bromine in the water. This also depends on how much Sodium Bromine is in the water. To get rid of the Sodium Bromine simply stop adding chlorine and let it burn off. pH Balance does not alter the effectiveness of Sodium Bromine as a sanitizer and oxidizer as it does for chlorine, however if you have both chlorine and Sodium Bromine in the water it is best to keep your pH Balance at the recommended level of 7.4 to 7.6. Sodium Bromine may turn yellow for a couple of minutes when introduced into water with chlorine present and a low pH Balance. This effect is harmless and will dissapate after a short while. Always be sure to resupply your sanitizer and oxidizer, mainly the chlorine, the week after Sodium Bromine is added as most of the Sodium Bromine will have broken down and the chlorine will have been depleted. In most cases, after this Sodium Bromine algeacide treatment you can return to your normal chlorine sanitation and oxidation system and maintain proper water chemistry levels to prevent future algae growth. Phosphate Removers as an Algaestat: What are Phosphates? Phosphates are microscopic dead plant material and provide algae with a ready food source. Chlorine is poisonous to algae, and Phosphates are algae's food. Just like a human with bad nutrition is more sensitive to disease and poison, so is algae. The combination of starvation and poisoning keep algae from ever getting started. Green algae are the worst threat to your pool water. While Mustard (yellow) Algae and Black (blue-green) Algae can make your pool surface unattractive, it's Green Algae that can consume your entire pool making it unsuitable for swimming. If Green Algae thrives long enough, little algae eating bugs will invade the pool. Soon after the bugs comes the bug eating frogs. If you let it go long enough, your pool water will return to its natural ecosystem form, primarily a pond. Swampy pools also become a breeding ground for disease carrying mosquitoes and you definitely do not want that. The essentials of algae life are food and shelter. For food algae needs water with phosphates. Without phosphates, even if Green Algae blooms, it won't really thrive, and it won't be healthy enough to survive chlorination. Shelter for algae is consistently wet surfaces with a decent chemical composition. Although Green Algae prefers surfaces such as your pool's walls and bottom, in a true Green Algae infestation it can inhabit even the water itself. In a typical situation, chlorinated water makes for lousy algae shelter. An extremely phosphate-rich pool generates healthy and hearty algae. These algae can sometimes survive extreme chlorine shocking, especially if the shocking comes in a series of less than totally destructive waves. Over time the algae can become chlorine Did you know? resistant. So it's clear that Phosphates have a major impact on Once algae has bloomed Green Algae or lack thereof. The next question is… it has consumed all the Phosphates it needs to live out its entire life. Using a Phosphate remover will not kill existing algae, however it will help to prevent future algae growth. It is what is called an Algaestat, and not a true Algaecide. Where do Phosphates come from? Phosphates are everywhere. Your tap water has significant amounts of Phosphates, so draining and refilling would not necessarily get rid of Phosphates. Phosphates wash off of the swimmers in your pool. Detergents and fertilizer contain huge amounts of Phosphates. Try to be very careful that ground water 33 doesn't run into your pool. There are even Phosphates in Liquid Chlorine. Organic decomposition is one of the largest sources of Phosphates. When a leaf falls into your pool and decomposes, guess what's produced? Phosphates. Every leaf, pine needle, pollen, every lizard, every mouse, every stick, berry or nut….every piece of plant or animal life remaining in the pool long enough to begin decomposing produces Phosphates. It's bad enough that such decomposition produces particulate matter to clog up your filter, but throw in the algae feeding Phosphates produced, and you can see that keeping your pool regularly cleaned is a must. The average pool will see an increase of Phosphate levels of about 50 ppm per week. Cannibalistic Algae? Decomposing algae is also organic matter that produces Phosphates. That’s right! You shock the pool to kill all the algae, and the dying algae create Phosphates to feed future algae blooms. How do Phosphate Removers Work? Phosphate removers lock up the Phosphates into a suspended solid that is filtered out by your filter. As the filter clogs with the suspended solid, backwashing and sanitizing the filter will remove them from your pool’s system. Each brand of Phosphate remover gives recommendations for how much remover to use based on the Phosphate reading of your water and the gallon capacity of your pool. Keep in mind that if you've been heavily shocking your pool, high chlorine levels can make it impossible to get an accurate reading on your Phosphate level. Also, if your pool is currently green, any measurement will show lower than what it would be after shocking the algae to death. In other words, it's very hard to get an accurate assessment of Phosphate levels in a problem pool. Phosphate Remover Marketing Before cleaning a filter it is strongly recommended to offer the customer a Phosphate removal treatment. The filter will have to be cleaned anyhow after a Phosphate removal, so since it is already due to be cleaned you might as well offer the additional service to improve the water’s chemistry and to also offer a profitable service. In cases of very high amounts of Phosphates, after using a Phosphate remover the Phosphates will clump up and fall out of circulation, causing a wide spread slimy coating all over the bottom of the pool. Vacuuming up this Phosphate fall-out will clog your filter, increasing the filter pressure very quickly and backwashing will be required to clear it out of your filter to be able to resume vacuuming. If your pool has a multi-port backwash valve that allows you to vacuum directly to waste, bypassing the filter, this would be strongly recommended. There are a large number of Phosphate removers available on the retail market. Phos-Free and Starvers are only a couple in a large line up to be mentioned here. Always be sure to read the label carefully and follow the directions on the label for best results. Most Phosphate removers are added through the skimmer after the filter has been back washed, however each has its own directions and recommendations. 34 Things to Remember About Section 10 An “algaecide” kills algae while an “algaestat” prevents algae. There are three primary categories of algaecides: Quats, Polyquats and Copper Salts. There are three types of metals used as algaecides in the current market. They are zinc, copper and silver. A chelating agent is an agent that prevents metals from falling out of circulation in the water and staining the surface. Most metal based algaecides claim to have some form of chelating agent although staining, when improperly applied and used does still occur. Sodium Bromine is a sanitizer and oxidizer just like chlorine is. Sodium Bromine is not dependent upon a particular pH Balance in order to sanitize and oxidize the way that chlorine is. Sodium Bromine is not protected by Conditioner the way that chlorine is so as a regular sanitizer and oxidizer it should only be used in indoor pools or covered spas, however it does make for an effective algaecide in outdoor chlorine pools and spas. Phosphates are microscopic dead plant material and provide algae with a ready food source. Phosphates are everywhere. Organic decomposition is one of the largest sources of Phosphates. The average pool will see an increase of Phosphate levels of about 50 ppm per week. Phosphate removers lock up the Phosphates into a suspended solid that is filtered out by your filter. As the filter clogs with the suspended solid, backwashing and sanitizing the filter will remove them from your pool’s system. 11. Treatments for Algae What do you do when algae begins to grow? That is a complex question, actually. Algae can have many different degrees of severity, there are many types of algae and many different conditions can cause it. It could be a little bit of Mustard (yellow) Algae in its infancy just starting to grow in the grout inside the skimmer or along the light fixture ring. It could be a pool completely overrun with Green Algae so thick that you can’t even see the top step and mosquitoes are swarming across the surface. It could even be the dreaded Black Algae, the black plague and scourge of swimming pool technicians and pool owners everywhere. There is a saying in the industry that goes “Ask ten different pool guys and you’ll get eleven different answers”. Written here are a few methods and techniques on how to get rid of algae in the field, though each swimming pool technician is different, will have different views and opinions and may agree or disagree with some or all of these methods and techniques. 35 Step 1: The first thing you want to do when algae begins to grow is find the breakdown in the three keys of healthy water (see section 3 Three Keys to Clear, Clean and Healthy Water). Is the algae caused by a lack of circulation, a lack of filtration, unbalanced chemistry or several of these keys? Is the pool pump operating for 8 hours each day? The pool should be circulating and filtering for 8 hours each day. Is the filter clean and has it been backwashed or sanitized recently? Make sure your filter is clean. Is the water’s chemistry balanced? The Free and Available Chlorine (FAC) should be at 3.0 to 5.0 ppm (parts per million). The water’s pH Balance should be at 7.4 to 7.6. The water’s Total Alkalinity (T.A.) should be between 80 and 120 ppm (parts per million). Is there enough Conditioner in the water? The Conditioner level, in order to protect the chlorine should be between 70 and 90 ppm (parts per million). How old is the water? The Calcium Hardness should be between 180 and 500 ppm (parts per million). Find the weakness or break in these three keys to healthy water. Perhaps the pH Balance got too high and the chlorine became ineffective, allowing the algae to grow. Perhaps there was not enough Conditioner in the water to effectively protect the chlorine. If the chlorine level drastically bounces during the week, being strong the day it is added and weak after a couple of days due to lack of protection, the algae can grow during these weak points and build up a resistance to the chlorine while it is weak. Step 2: Brush the walls and floor down with a wall brush, a steel wire wall brush in the case of Black Algae or very strong Mustard (yellow) Algae or Green Algae. Brushing the walls and floor will break up the algae, knocking off the blooms or flowers and expose them to the chemicals. Remember to NEVER use a steel wire wall brush on a fiberglass, painted or vinyl lined pool. In the case of Black Algae, scrubbing it with a steel wire brush will score (scratch) the surface of it, much like a cut or scrape on a human being, exposing it to infection or in the case of water chemistry exposing it to the sanitizers, oxidizers and algaecides. Step 3: Adding the chemicals can be complex as different amounts are added depending on the severity and type of algae you are trying to kill. Here I will be general when describing applications of chemicals, however know that you should add more chemicals with larger sized pools and more severe algae conditions. 1. As safely as possible, lower the pH Balance using Muriatic Acid. If you can do so safely, bring the pH Balance to 7.3 to 7.4. Why? This will keep the chlorine in its strongest possible killing form without making the water acidic and corrosive. 2. Bring the chlorine up to at least 5.0 ppm (parts per million) or higher. Why? Even though by this time the algae is likely very resistant to the chlorine it will make sure that the pool has plenty of sanitizer and oxidizer available to keep the Sodium Bromide charged when it is later added. 3. Add 2 lbs. of Potassium Peroxymonopersulfate per 10,000 gallons of water. 36 Why? The Potassium will oxidize any organic material in the water, leaving your chlorine free to sanitize, making the chlorine more effective. 4. Add 6 to 8 ounces of Sodium Bromide per 10,000 gallons of water. Be sure the Sodium Bromide is at least 98% active ingredient. Why? The Sodium Bromide is also a sanitizer and oxidizer but it is one that the algae has not yet experienced so it will not have built up any resistance to it. The high level of chlorine in the water will keep the Sodium Bromide charged and working very effectively. The combination of chlorine in low pH conditions, Potassium Peroxymonopersulfate and Sodium Bromide used as an algaecide will be enough to burn out just about any form of algae. Black Algae may take repeated applications as well as brushing the walls and floor with a steel wire brush as often as possible to keep the algae scored (scratched) and exposed to the chemicals. In the case of a pool completely overrun with Green Algae, super chlorination may be required as well which could require double, even triple the normal amounts of chlorine. NEVER use a steel wire wall brush on a fiberglass, painted. tiled or vinyl lined surface. Step 4: The following week, when the algae has cleared up make sure your filter is backwashed and cleaned and your pump is running for 8 hours per day. Be sure to replenish the sanitizer and oxidizer to the water (chlorine) as the chlorine and Sodium Bromide will likely have been depleted. Step 5: It is a good idea to test for Phosphates in the water and add a Phosphate remover accordingly, as an abundant source of Phosphates can be a catalyst for algae to quickly return and dead algae also becomes Phosphates for future algae spores to eat. When dealing with Black Algae be sure to rinse any wall brushes or steel wire brushes off with liquid chlorine after use as Black Algae can be transferred from one pool to another if you are not careful. Maintain the three keys to healthy water…circulation, filtration and chemical balance to help prevent algae from returning. It may return now and again but a good technician will see it and treat it quickly before the home owner can see it with the naked eye. Here are some charts to be used as guide lines when removing algae: Notes Regarding these Charts Chlorine on these charts refers to either granular chlorine such as Dichlor or Liquid Chlorine. Chlorine is represented by lbs/gals meaning pounds or gallons, whichever you are using Other forms of granular chlorine may effect the pH Balance such as Trichlor (Algae Ban) having a very low pH or Calcium Hypochlorite having a fairly high pH Conditioner on these charts refers to granular Conditioner 37 How to Treat Mustard (Yellow) Algae, (Mild Cases) For mild cases of mustard (yellow) algae just beginning, such as a little bit forming in the grout within the skimmer, the corners and around the light fixture or very light amounts beginning to form on the wall, treat as follows: 10,000 Gallons 15,000 Gallons Add: 2 lbs/gals chlorine Add: 2-3 3” Trichlor tablets to a float dispenser or chlorinator Add: 8 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs Conditioner will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4 20,000 Gallons Add: 3 lbs/gals chlorine Add: 2-3 3” Trichlor tablets to a float dispenser or chlorinator Add: 10 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs conditioner will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4 25,000 Gallons Add: 4 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs granular Conditioner will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 38 Add: 5 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons 35,000 Gallons Add: 6 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. Add: 7 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. How to Treat Mustard (Yellow) Algae, (Bad Cases) For bad cases of mustard (yellow) algae, such as algae coverage over an entire wall or floor, treat as follows: 10,000 Gallons 15,000 Gallons Add: 4 lbs/gals chlorine Add: 3 3” Trichlor tablets to a float dispenser or chlorinator Add: 10 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Add: 5 lbs /gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. 39 Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lb/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 25,000 Gallons Add: 6 lbs /gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 ½ lbs will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons Add: 7 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 ¾ lbs will raise the conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 9 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals of chlorine and make sure the pH balance remains at Add: 10 lbs/gals chlorine Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains 40 7.3 – 7.4. at 7.3 – 7.4. How to Treat Mustard (Yellow) Algae, (Severe Cases) For severe cases of mustard (yellow) algae, such as algae wide spread coverage over walls, floors and deep, dark, almost orange algae that doesn’t brush off, treat as follows: 10,000 Gallons 15,000 Gallons Add: 8 lbs/gals chlorine Add: 1 lbs Potassium Monopersulphate Add: 4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a steel wall brush (except for fiberglass, vinyl lined or complete tile surfaces) Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. Add: 10 lbs/gals chlorine Add: 1 1/2 lbs Potassium Monopersulphate Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a steel wall brush (except for fiberglass, vinyl lined or complete tile surfaces) Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons 25,000 Gallons Add: 12 lbs/gals chlorine Add: 2 lbs Potassium Monopersulphate Add: 5 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a steel wall brush (except for fiberglass, vinyl lined or complete tile surfaces) Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Add: 14 lbs/gals chlorine Add: 2 1/2 lbs Potassium Monopersulphate Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1¾ lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a steel wall brush (except for fiberglass, vinyl lined or complete tile surfaces) Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. 41 Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 16 lbs/gals chlorine Add: 3 lbs Potassium Monopersulphate Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a steel wall brush (except for fiberglass, vinyl lined or complete tile surfaces) Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. Add: 18 lbs/gals chlorine Add: 3 1/2 lbs Potassium Monopersulphate Add: 8-10 3” Trichlor tablets to a float dispenser or chlorinator Add: 22 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a steel wall brush (except for fiberglass, vinyl lined or complete tile surfaces) Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. How to Treat Green Algae, (Mild Cases) For mild cases of green algae just beginning, such as a little bit forming in the grout within the skimmer, the corners and around the light fixture or very light amounts beginning to form on the wall, treat as follows: 10,000 Gallons 15,000 Gallons Add: 2 lbs/gals chlorine Add: 2-3 3” Trichlor tablets to a float dispenser or chlorinator Add: 8 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm 42 Add: 3 lbs/gals chlorine Add: 2-3 3” Trichlor tablets to a float dispenser or chlorinator Add: 10 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 25,000 Gallons Add: 4 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons Add: 5 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 6 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine Add: 7 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals and 43 and make sure the pH balance remains at 7.3 – 7.4. make sure the pH balance remains at 7.3 – 7.4. How to Treat Green Algae, (Bad Cases) For bad cases of green algae, such as algae coverage over an entire wall or floor, treat as follows: 10,000 Gallons 15,000 Gallons Add: 4 lbs/gals chlorine Add: 3 3” Trichlor tablets to a float dispenser or chlorinator Add: 10 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons Add: 6 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 25,000 Gallons Add: 7 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine Add: 10 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals 44 and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 12 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. Add: 14 lbs/gals chlorine Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 8898%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. How to Treat Green Algae, (Severe Cases) For severe cases of green algae, such as strong total algae coverage over entire walls, floors and green water treat as follows: 10,000 Gallons 15,000 Gallons Add: 6 lbs/gals chlorine Add: 3 3” Trichlor tablets to a float dispenser or chlorinator Add: 10 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm 45 Add: 9 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 25,000 Gallons Add: 12 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons Add: 15 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 18 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine Add: 20 lbs/gals chlorine Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals 46 and make sure the pH balance remains at 7.3 – 7.4. chlorine and make sure the pH balance remains at 7.3 – 7.4. How to Treat Green Algae, (Swamp) For water that has become a complete swamp, such as can no longer see the top step, mosquitoes in the water, completely green from top to bottom treat as follows: 10,000 Gallons 15,000 Gallons Add: 12 lbs/gals chlorine Add: 3 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. Add: 15 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons 25,000 Gallons Add: 18 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Add: 21 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. 47 Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 24 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals and make sure the pH balance remains at 7.3 – 7.4. Add: 27 lbs/gals chlorine Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 22 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae with a regular wall brush. Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. How to Treat Black (Blue-Green) Algae, (Regular Cases) For regular cases of black (blue-green) algae, such as small dime or smaller sized, rounded, spotted, dark slimy growths in a few areas such as the corners and walls treat as follows: 10,000 Gallons 15,000 Gallons Add: 8 lbs/gals chlorine Add: 3 3” Trichlor tablets to a float dispenser or chlorinator Add: 10 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm 48 Add: 10 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 25,000 Gallons Add: 12 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons Add: 15 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 35,000 Gallons Add: 18 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile 49 Add: 20 lbs/gals chlorine Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. How to Treat Black (Blue-Green) Algae, (Severe Cases) For severe cases of black (blue-green) algae such as nickel or smaller sized, rounded, spotted, dark slimy growths in many patchy areas throughout the surface treat as follows: 10,000 Gallons 15,000 Gallons Add: 12 lbs/gals chlorine Add: 3 3” Trichlor tablets to a float dispenser or chlorinator Add: 12 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). ¾ lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 20,000 Gallons Add: 15 lbs/gals chlorine Add: 3-4 3” Trichlor tablets to a float dispenser or chlorinator Add: 14 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 2 lbs/gals and make sure the pH balance remains at 7.3 – 7.4. 25,000 Gallons Add: 18 lbs/gals chlorine Add: 4-5 3” Trichlor tablets to a float dispenser or chlorinator Add: 16 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile 50 Add: 21 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 18 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 1 3/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 3 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. 30,000 Gallons 35,000 Gallons Add: 24 lbs/gals chlorine Add: 5-6 3” Trichlor tablets to a float dispenser or chlorinator Add: 20 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/4 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 4 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. Add: 27 lbs/gals chlorine Add: 6-8 3” Trichlor tablets to a float dispenser or chlorinator Add: 22 oz Sodium Bromine based granular algaecide (preferably as pure as possible, 88-98%, such as YellowTreat or YellowTrine) Add: Muriatic acid enough to lower the pH balance to 7.3 (The amount to add will depend on the current pH balance and the Total Alkalinity of the water) Add: Conditioner to raise the level to 70-90 ppm (parts per million) to protect the chlorine. (The amount added will depend on the current conditioner reading). 2 1/2 lbs will raise the Conditioner 10 ppm Brush: the walls, floor and any place there is algae rigorously and daily if possible with a steel wire wall brush (except for fiberglass, vinyl lined or all tile surfaces). Next week: The chlorine will likely be gone, consumed by the Sodium Bromine algaecide. Replenish the chlorine by adding 5 lbs/gals chlorine and make sure the pH balance remains at 7.3 – 7.4. You Might Have Noticed On these charts the amounts of chemicals used, particularly for the more severe cases of algae in larger pools can be staggering. You need a proportionate amount of chemicals to combat an equally proportionate amount of algae. Think of the chemicals like a paper towel to clean up a spill. The algae and size of the pool are like the size of the spill and over how much area the spill covers, however keep in mind that the paper towel only holds so much. A sigle paper towel used to clean up a gallon of liquid is not even close to enough. With this in mind, if you do not use enough chemicals to completely remove the problem than after your chemicals are depleted you will not only still have unsightly algae filled water remaining but the algae will now also be resistant to the chemicals that you used as it has survived your first treatment. Also, in cases where large amounts of chlorine are called for, you may want to consider using a less expensive form of dry chlorine such as Calcium Hypochlorite. It isn’t the healthiest form of chlorine, however it gets the job done, is lighter and easier to carry than 20 or more gallons of liquid chlorine and is far less expensive, pound for pound than using Dichlor. Remember from section 2…the right tool for the right job. This is an effective maintanance program to help prevent algae formation: 51 The “Four Pillars” Algaestat/Algaecide Regular Maintanance Program First Pillar Second Pillar Three Keys to Clean, Clear and Healthy Water. Always maintain your cilrculation, filtration and proper chemical balance. Chlorine maintained at 3 to 5 ppm using an immeditate for of chlorine as well as a back up slow dissolve chlorine such as 3” Trichlor tablets within a pH Balance of 7.4 to 7.6 will provide your water with ample sanitation and oxidation power. Keeping the Conditioner level at 70 to 90 ppm at all times will keep the chlorine properly protected. Plenty of circulation and filtration will keep the water clean and clear from organic and oxidized debris. Using a slow dissolve metal based and chelated algaecide such as Nature2 or the PoolRX, particularly in the Spring and Summer months will add an additional and more powerful form of sanitation to the water which will aid in more efficiently and effectively killing germs and bacteria and will also free up the chlorine for oxidizing organic material. *This special service should be charged extra to the customer. Third Pillar Fourth Pillar Using Sodium Bromine in small amounts (2 ounces of granular or 2 squirts from liquid) on a weekly basis, particularly in the Spring and Summer months will consume a small amount of the existing chlorine, however it will provide the water with a secondary sanitizer and oxidizer that is not dependent on the pH Balance. Chlorine, Sodium Bromine and metals in the water at the same time will offer algae little to no chance of ever developing. *Algaecides should be charged extra to the customer. Using a Phosphate removal treatment as well as a small amount of Phosphate remover on a weekly basis, particularly in the Spring and Summer months will greatly reduce the amounts of Phosphates in the water. Without Phosphates, algae will be under nourished and starve being very frail if it is able to grow at all. *Phosphate removal treatment should be done before a filter clean and charged extra to the customer. Customers should also be sold additional Phosphate remover to be used on a weekly basis. 52 Things to Remember About Section 11 Algae can have many different degrees of severity, there are many types of algae and many different conditions can cause it. The first thing you want to do when algae begins to grow is find the breakdown in the three keys of healthy water (see section 3 Three Keys to Clear, Clean and Healthy Water). Brush the walls and floor down with a wall brush, a steel wire wall brush in the case of Black Algae or very strong Mustard (yellow) Algae or Green Algae. Brushing the walls and floor will break up the algae, knocking off the blooms or flowers and expose them to the chemicals. Adding the chemicals can be complex as different amounts are added depending on the severity and type of algae you are trying to kill. NEVER use a steel wire wall brush on a fiberglass, painted, tiled or vinyl lined surface. The following week, when the algae has cleared up make sure your filter is backwashed and cleaned and your pump is running for 8 hours per day. Be sure to replenish the sanitizer and oxidizer to the water (chlorine) as the chlorine and Sodium Bromide will likely have been depleted. It is a good idea to test for Phosphates in the water and add a Phosphate remover accordingly, as an abundant source of Phosphates can be a catalyst for algae to quickly return and dead algae also becomes Phosphates for future algae spores to eat. When dealing with Black Algae be sure to rinse any wall brushes or steel wire brushes off with liquid chlorine after use as Black Algae can be transferred from one pool to another if you are not careful. There are many products available and many techniques to treating algae. Don’t forget that algae growth will usually first be seen on the grout inside the skimmer, along the light ring and on any string that may tie a 3 inch Trichlor tablet floater in place. You will be able to see this algae with U.V. protected polarized Sun glasses before you can see it with the naked eye. NEVER just brush the algae away when you see it. Brushing the algae just relocates it and doesn’t solve the problem. You must treat the problem, not just hide a symptom else the algae will be more visible and likely chemical resistant the following week. 53 12. Salt Chlorination Systems A salt chlorination system is an electronic system that is plumbed into the swimming pool’s plumbing. While there are many different brands and styles of salt chlorination systems each follows the same basic principles. Each is made up of the same basic components and operates primarily the Did you know? same way. The idea is for this electronic system to Chlorine is a gas. Sodium Chloride, or pass salt, which is added to the pool water through a commonly known as salt has the chlorine series of metal panels that are contained within a salt gas trapped within a Sodium crystal. Even cell that is added onto the swimming pool’s plumbing. when dissolved in water and unseen by the As this salt water is passed through these panels a naked eye it is still a crystal. Chlorine in its faint and safe electric charge is passed through the gas form has a very high pH Balance of cell’s panels. This electronic charge frees the chloride about 13 which means that once a salt from the Sodium Chloride (salt) and the chloride alone, chlorination system frees that gas chlorine now chlorine disperses into the swimming pool water. from the sodium crystal it has released a very high pH chlorine into the water. This is The system is controlled as to how much or how little why salt chlorination systems always seem chlorine is produced by a computer board. Most of to raise the pH Balance in swimming pool these salt chlorination systems are self cleaning, and spa water. although only to a certain degree and still require regular maintenance and inspection. Most of these salt chlorination systems will also have other functions such as telling you the amount of salt in the water, if the salt cell is dirty and needs cleaning as well as a multitude of other self diagnostic readings. Salt chlorination systems have become increasingly popular as swimmers feel a much “softer” feeling saline solution of water and do not notice the chemical feel or smell of the chlorine, even though it is still chlorine being produced. There are many pros and cons to using a salt chlorination system which we will cover here. The Pros of Using a Salt Chlorination System: 1. The water feels “softer” and more comfortable, like a mild saline solution to swimmers 2. Salt is much less expensive than chlorine, at about $ 4.00 to $ 4.50 per 50 lbs. bag 3. Swimmers do not notice the smell or effects of chlorine in the water as they might with a non-salt system pool 4. The chlorine produced can be regulated, keeping a much more stable amount of chlorine in the water 54 The Cons of Using a Salt Chlorination System: 1. The chlorine produced by a salt chlorination system is a very high pH product, having a pH Balance of about 13 and is produced while the pump is operating, about 8 hours per day. This means that the water requires much more acid than a regular pool to keep the pH Balanced, or otherwise known as a high acid demand 2. The high acid demand to balance the pH of the water can drastically increase the speed of which the Total Alkalinity is lowered because of the larger quantities and regularity of acid added 3. For a salt chlorination system to operate normally the average amount of salt needed in the water is 3250 to 3500 ppm (parts per million). This is a lot of additional Total Dissolved Solids (TDS) in the water, shortening the time needed to drain and refill the pool to keep the water healthy 4. Because of the increased Total Dissolved Solids (TDS) and the larger amounts of acid added there is a greater risk of staining to the surface from minerals and metals falling out of circulation. This additional staining will be particularly evident on white plaster surfaces 5. Salt is naturally corrosive to metals such as welds, stainless steel filters and copper plumbing. Only Fiberglass or other plastic filters should be used with a salt chlorination system and only when the pool has pvc plumbing. While there are many pools with copper plumbing that have been remodeled and a salt chlorination system has been added this practice is not recommended 6. While the salt cell is often self cleaning it will over time become coated with calcium. Keeping the pH balanced, the Total Alkalinity in balance and the Calcium Hardness fairly low will help reduce this calcium coating. The salt cell must still be inspected every 90 days and cleaned as needed 7. Each salt cell tends to have a varying life expectancy of 1 to 3 years on average depending upon brands and care of the water chemistry; however most of them have a warrantee and can be prorated as far as replacement costs and installation. Always check with the manufacturer and have the cell number and serial number handy. The salt cells cost upwards of $ 550.00 or more each 8. The salt chlorination systems is dependant upon its computer which is usually kept outside by the swimming pool equipment, exposed to the outdoor elements of rain, heat and cold temperatures. Even though they are often well insulated and protected they are susceptible to damage and are expensive to repair and replace 9. The salt chlorination system computer’s diagnostics, such as salt level, water flow and when the salt cell should be cleaned are dependent upon electronic readings. When a problem or discrepancy develops in the system it can often give false readings. Always test your salt levels independently using a salinity test strip or electronic salt meter to determine the amount of salt in the water 55 So, as you can see there are many more cons than pros of using a salt system, however swimming pool owners often love having them and clearly the salt chlorination system salesmen rarely, if ever tell them all of the cons of the system. Regardless of whether they are the best way to oxidize and sanitize a pool or not, these systems are out there and are growing in popularity so, as a technician we must be prepared to properly maintain them. Remember these few important notes about salt chlorination systems: Salt Chlorination System Note NEVER trust a salt chlorination system board for the salt content. These systems are VERY often out of calibration and give incorrect readings. If you add salt to the water based on an incorrect reading you could be adding way too much salt and causing damage to the salt chlorination cell and can cause mineral and metal fall out and surface staining. ALWAYS have a secondary source of testing the salinity level whether it be test strips or an electronic meter. On average each system will want you to maintain the salt level at 3250 to 3500 ppm (parts per million). Too much salt can increase the risk of burning out the salt cell. Be sure to do your own independent test of the salt level with a salt test strip or other testing device. The computer may not always be accurate and adding too much salt due to a false computer diagnostic reading can result in burning out the salt cell and you’ll have to partially drain the pool to get rid of the excess salt. Regularly inspect and clean the salt cell as needed every 90 days or as the manufacturer of the salt cell recommends Anticipate the pH Balance to increase much more so than a regular pool because of the high pH chlorine being produced when the salt chlorination system is running. Salt chlorination systems have a high acid demand and adding enough acid to keep the pH balanced will also lower the Total Alkalinity more rapidly than in a normal pool Adding Salt to the Pool: Did you know? When the salt cell self cleans it removes much, but not all of the calcium build up which collects on the metal panels inside the salt cell. This calcium is sent back into the pool through the return lines and can become very visible in the spa. The calcium will look like little slimy snow drifts. If you have an automated control system you can program the spa only to cycle for about 30 minutes per day to help remove this calcium build up. A 50 lbs. bag of salt will raise the salt level in an average sized 20,000 gallon pool about 300 ppm (parts per million), however always consult with a “salt per gallon” chart usually found on the back of a bag of salt. Always be sure to add less salt than required and slowly reach the desired level as to not accidentally add too much salt. I have found that the salt crystals are much more effective and disperse much more rapidly than the salt pellets. When adding the salt, some technicians recommend premixing the salt in a bucket of water and adding it after it dissolves, however to my own personal experience I have found no negative side effects in adding the salt directly to the pool water and brushing it evenly over the pool to help with more rapid dissolution. When adding salt to the pool be sure that the pool pump is operating and the water is circulating. 56 Inspecting and Cleaning the Salt Cell: Every 90 days you should unscrew and inspect the salt cell. First, make sure the pump is turned off and it is a good idea to place the filter in its “backwash” setting (For D.E. style filters). The reason for this is that since we will be opening up the plumbing by unscrewing the salt cell we do not want the filter to drain down back into the pool, just in case it does not have a check valve to stop such an occurrence. You can use a large set of channel locks to open the unions on the cell to unscrew it. There are rubber o-rings in place where the unions are threaded in, so be sure that these o-rings do not fall out of place. The rubber o-rings keep the salt cell sealed and from leaking water. Once the salt cell is open look inside of it. If the metal panels inside are clean and free from any white calcium build up, than screw the unions back into place, remembering to hand tighten only, although I always give it just a little extra tightening with my channel locks just to make sure they are on there securely but very carefully so to not over tighten them or risk breaking the plastic unions. Put your pool filter back Salt Cell Note into its regular circulation mode, out of “backwash” and turn the The average salt pump on to make sure there are no leaks at the unions. Inspect chlorination cell has a life the salt cell again after another 90 days. span of about 2 years. Some Salt Chlorination System brands will have a warrantee period and will prorate the cost of a new cell to the customer. If you inspect the salt cell and find that the metal panels within are caked in calcium, which will be a white slimy material almost like wet chalk, than you’ll need to clean the salt cell. There are a few ways of cleaning a salt cell and often different technicians and even warrantee technicians for these salt chlorination systems recommend different ways. 1. Use a long stick, brush or similar tool to flick the larger chunks of calcium off the panels. Most of this calcium will scrape right off, however be careful as to not damage the panels themselves. 2. There are fittings made with unions just for cleaning a salt cell. These fittings can be found at most any swimming pool supply store or also try contacting the factory representative for that particular brand of salt chlorination system. They will often be happy to send you the fittings for cleaning these cells and some will even meet you there at the account to instruct you in how to clean it. These fittings screw onto the salt cell in the same way in which it screws onto the pool plumbing, however the ends of these fittings are enclosed in pvc and act as reservoirs to hold the cleaning solution that you will use to clean the cell. 3. If these fittings are not available you can use a bucket, holding the salt cell down vertically in the bucket, enclosing the bottom of the cell as to not allow the cleaning solution to escape. 4. The cleaning solution is composed of 3 parts water and 1 part Muriatic Acid. For a stronger cleaning some technicians may recommend an even 50/50 mix of water to acid, however when using such chemicals it is always important to consider safety. You can also add a half of a cap full of “Plaster White and Brite”, a form of Flouridic Acid used to keep the fumes down during an acid wash. 57 5. Using this cleaning solution, pour the solution into the salt cell, only on the inside if it is possible to keep it there with the salt cell cleaning fittings or by using a bucket. Be sure to have a garden hose handy in case of any spills or accidents and also for rinsing out the salt cell from the cleaning solution once all of the calcium has been dissolved away. The solution should bubble and fizz a bit as it dissolves away the calcium. The process should take only a couple of minutes. Caution! 6. Rinse off the salt cell with water and around your work area to make sure there is no spilled or left over acid anywhere, then screw the salt cell back onto the plastic unions on the pool’s plumbing Always be careful when you are cleaning out a salt cell. In case of a spill or over flow you want to be in a location that is easy to 7. Hand tighten the unions to make sure the salt cell is clean such as over dirt or gravel. tightened and secure, place the “backwash valve” back A spill over the customer’s deck into its normal circulation setting and turn the pump on can be a costly mistake should it to make sure there are no leaks. Allow the pump to corrode and stain the deck. Have operate for a few minutes before checking the salt cell a bucket of water handy just in diagnostic readings to make sure the salt chlorination case of a spill. system is functioning normally. Your cell is now clean and you should inspect it again in 90 days. Additional notes about Salt Chlorination Systems: When the salt cell needs cleaning, the pH balance is too high, the Total Alkalinity is too high or the Calcium Hardness is too high you will start to see residual amounts of calcium on the pool and spa surface. You will primarily see this calcium in the spa and it will look like small snow drifts made from this softened calcium from the salt cell. Cleaning the salt cell and checking and balancing your water chemistry will fix this. The salt cell is usually self cleaning. It will have two cycles, though each is made and may function differently. The cell, in most cases will have a production cycle and a cleaning cycle. During this cleaning cycle it will often clean off this residual calcium build up on the panels which will end up back in the water on the surface. This is also where these snow drifts of soft, slimy calcium in the spa come from. Even though the water seems very soft and user friendly to swimmers, the product being created in the water is still chlorine. It is advised that swimmers, just as with a regular chlorine pool rinse off, at least their face before entering the water to remove any excessive organic material such as sweat, dead skin cells and other human waste, else the chlorine will bond with that organic material and form uncomfortable and foul smelling chloramines. A pool with a salt chlorination system should be drained and refilled once every 3 years, rather than once every 3 to 5 years (Depending on the Calcium Hardness and Total Dissolved Solids levels) to help reduce the risk of staining. 58 Salt chlorination systems can be a great source of chlorine, however they require more maintenance and constant chemical balance to operate. The water seems more comfortable for swimmers as the chemicals are well camouflaged in 3500 ppm (parts per million) of salt in the water. Ideally I would recommend a salt chlorination system for a “Pebble Tech” surfaced pool as the resulting staining will not be seen, however for other pool surfaces the added risk of staining is substantially increased over time. There are many pros and cons to having a salt chlorination system and they are often debated among technicians and manufacturers. In the end, swimming pools owners seem to prefer these systems and are often sold on the pros of these systems while being unaware of many of the cons. Things to Remember About Section 12 The idea of a salt chlorination system is to pass salt, which is added to the pool water through a series of metal panels that are contained within a salt cell that is added onto the swimming pool’s plumbing. As this salt water is passed through these panels a faint and safe electric charge is passed through the cell’s panels. This electronic charge frees the chloride from the Sodium Chloride (salt) and the chloride alone, now chlorine disperses into the swimming pool water. Salt chlorination systems have become increasingly popular as swimmers feel a much “softer” feeling saline solution of water and do not notice the chemical feel or smell of the chlorine, even though it is still chlorine being produced. For a salt chlorination system to operate normally the average amount of salt needed in the water is 3250 to 3500 ppm (parts per million). The salt cell must still be inspected every 90 days and cleaned as needed. Always test your salt levels independently using a salinity test strip or electronic salt meter to determine the amount of salt in the water Anticipate the pH Balance to increase much more so than a regular pool because of the high pH chlorine being produced when the salt chlorination system is running. Salt chlorination systems have a high acid demand and adding enough acid to keep the pH balanced will also lower the Total Alkalinity more rapidly than in a normal pool A 50 lbs. bag of salt will raise the salt level in an average sized 20,000 gallon pool about 300 ppm (parts per million), however always consult with a “salt per gallon” chart usually found on the back of a bag of salt. The cleaning solution is composed of 3 parts water and 1 part Muriatic Acid. For a stronger cleaning some technicians may recommend an even 50/50 mix of water to acid, however when using such chemicals it is always important to consider safety. Even though the water seems very soft and user friendly to swimmers, the product being created in the water is still chlorine. It is advised that swimmers, just as with a regular chlorine pool rinse off, at least their face before entering the water to remove any excessive organic material such as sweat, dead skin cells and other human waste, else the chlorine will bond with that organic material and form uncomfortable and foul smelling chloramines. 59 13. About Swimming Pool Filters There are three different styles of filters commonly used for swimming pool filtration. The regulations for what filters can be used for a residential or commercial swimming pool change from city to city and state to state, however you will find all three types just about everywhere you service swimming pools.The three types of filters we will discuss here are Diatomaceous Earth Filters, Cartridge Filters and Sand Filters. We will also discuss the principles of how they work and their maintenance. Diatomaceous Earth Filters: Caution! Diatomaceous Earth (D.E.) Filters can be metal, fiberglass or plastic just as all other filters. Water is pushed through a D.E. filter, just as any other filter. The pump pushes the water into the filter past a series of 8 D.E. filter grids. These grids have a plastic skeleton and are coated in a fine screen-like mesh material. This material is made to hold a filter media called Diatomaceous Earth (D.E.) which is also commonly called filter powder. It is a very fine powder, much like baby powder. The filter grids job is to hold the powder, while water is pushed through and inside the filter grids and then returned to the pool. Any dirt and debris is held by the D.E. powder while the clean and filtered water is returned to the swimming pool. Diatomaceous Earth (D.E.) is a known carcinogen and known to cause cancer. If it is breathed in, D.E.’s particles are so fine that they can become trapped inside the lungs. Always use caution when handling D.E.. What is Diatomaceous Earth? “Diatomaceous earth (pronounced /ˌdaɪətəˈmeɪʃəs ˈɝθ/), also known as DE, TSS, diatomite, diahydro, kieselguhr, kieselgur or celite) is a naturally occurring, soft, chalk-like sedimentary rock that is easily crumbled into a fine white to off-white powder. This powder has an abrasive feel, similar to pumice powder, and is very light, due to its high porosity. The typical chemical composition of diatomaceous earth is 86% silica, 5% sodium, 3% magnesium and 2% iron. Diatomaceous earth consists of fossilized remains of diatoms, a type of hard-shelled algae. It is used as a filtration aid, as a mild abrasive, as a mechanical insecticide, as an absorbent for liquids, as cat litter, as an activator in blood clotting studies, and as a component of dynamite. As it is also heat-resistant, it can be used as a thermal insulator. Filtration The most common use (68%) of diatomaceous earth is as a filter medium, especially for swimming pools. It has a high porosity, because it is composed of microscopically-small, coffin-like, hollow particles. It is used in chemistry under the name Celite as a filtration aid, to filter very fine particles that would otherwise pass through or clog filter paper. It is also used to filter water, particularly in the drinking water treatment process and in fish tanks, and other liquids, such as beer and wine. It can also filter syrups and sugar. Other industries such as paper, paints, ceramics, soap and detergents use it as a fulling material.” - Wikapedia 60 When a D.E. filter grid’s mesh material is damaged it allows the D.E. powder and any other dirt and debris to travel through the tear in the mesh and return back into the pool. Diatomaceous Earth is added to the filter through the skimmer while the pump is operating. It mixes with the water inside the skimmer and within the plumbing from the skimmer to the filter where, once inside the filter it coats the filter grids. Add 1 lbs. of D.E. (1 filled orange D.E. scoop) per each 10 square feet of filtration area of the filter. For example a 60 sq. ft. D.E. filter would require 6 lbs. of D.E. in order to function and filter properly. The 1 lbs. D.E. Scoop The 1 lbs. D.E. Scoop is usually a bright orange color when brand new and useful for all sorts of things such as carrying other dry chemicals to the pool, carrying As the filter collects dirt and debris the pressure within water to prime a pump or to rinse off the filter gets higher. Every month or two depending on after a salt cell cleaning and even to put how much debris the filter is holding and its filter at the bottom of a skimmer to stop the pressure, the filter should be backwashed. When a filter suction in cases where there are 2 is backwashed it changes the direction of water flow and skimmers plumbed into the same instead of the water flowing through the grids and back suction line. This is a handy trick to into the pool it is redirected to flow from within the grids gaining full suction at the other skimmer blowing outward and returns to the backwash line, when there is no valve present to divert usually to the sewer line or P-trap. Flowing out the filter suction. grids instead flowing into them blows the dirt and debris filled D.E. powder off of the grids. Backwashing the filter will get most of the old D.E. and dirt out of the filter but not all of it. Did you know? “In 1866, Alfred Nobel discovered that nitroglycerin could be made much more stable if absorbed in diatomite. This allows much safer transport and handling than nitroglycerin in its raw form. He patented this mixture as dynamite in 1867, and the mixture is also referred to as guhr dynamite.” – Wikapedia That’s right! Diatomites are not just useful for the filtration or your D.E. filter but are also used to make dynamite. Every 6 months a D.E. filter should be taken apart and cleaned manually by removing and hosing off all the grids individually, making sure each is thoroughly inspected for tears or damage and cleaned, rinsing it off with a garden hose. This is generally called a “filter sanitation”. Once the filter has been inspected and sanitized, reassemble it. Make sure the filter tank rubber oring has been lubricated with a Teflon based lubricant. “Magic Lube” is an excellent brand of Teflon based lubricant used for rubber o-rings. Once the filter is back in its normal operating mode, replace the D.E. powder with fresh D.E. adding it through the skimmer. To backwash a D.E. filter there will be either a push/pull style of handle, a multi-port type of handle or a metal bar at the bottom of the filter that needs to be moved into the opposite direction. It is recommended to get some instruction from a professional if you have never sanitized a filter, before attempting to do it yourself. Hands on experience can be an excellent teacher and this guide is here only to teach the basic principles of a D.E. filter. 61 Further Notes Regarding Diatomaceous Earth Filters: To backwash a D.E. filter first make sure the pump is off. Next find out where the backwash line goes to. It should go to a sewer line which will likely be alongside the house somewhere, near the pool equipment or even in the front yard. If there is a P-trap (a question mark shaped plumbing line that directs water down into a larger line which then travels to the sewer) than be sure to check for and remove the “rodent screen” before backwashing, else the water will overflow past the P-trap and could spill into the yard. Next move the backwash valve into the backwash position. This valve could be a push/pull valve, a multi-port valve or a metal bar protruding out horizontally from the bottom or the filter. Turn the pump back on and the filter will be in its backwash cycle. Allow it to backwash for about 60 seconds. Once the filter is backwashed or sanitized put the backwash valve back into the pool circulation or filter setting. Turn the pump back on. When adding Diatomaceous Earth through the skimmer to recoat the filter grids be careful to not breathe in the powder as it can be hazardous to your health. Add 1 lbs. of D.E. powder per each 10 sq. ft. of the filtration area. Once you add the D.E. powder through the skimmer, carefully, one scoop at a time, check the return lines in the pool and spa. If you see any D.E. returning to the pool than you have a problem inside the filter. This problem could be a tear in one of the filter grids or a missing o-ring. This problem could also occur if the filter was reassembled incorrectly. If you do not see any D.E. powder returning through the return lines than your filter is working properly. When doing a filter sanitation it is good to replace the filter tank o-ring about once per year. When you replace the filter tank o-ring be sure to have the correct replacement o-ring or else it may not fit properly or seal the tank. You do not want to have any leaks on the tank as that would cause the swimming pool to lose water and the filter to drain down and empty when the pump turns off. Always have the brand, style and size of your filters documented so when you purchase a new filter tank o-ring or other filter parts you are sure to purchase the correct parts. When sanitizing a filter be sure to clean up any mess and do not hose off the filter grids in a place that would make a mess of the customer’s yard. This would be seen as unprofessional and careless. Check the P-trap regularly when the filter is in its normal operating mode to make sure that the backwash valve is not leaking water into the sewer line. Cartridge Filters: Cartridge filters are very similar in function, shape and size to D.E. filters. Often a manufacturer will use the same body of a D.E. filter and only change the insides to hold a cartridge or several cartridges rather than D.E. filter grids. Cartridge filters operate in a similar manner as D.E. filters in 62 that the pump pushes water through them, however the difference is in that rather than having grids to hold D.E. powder, within the filter is a large cartridge or a series of smaller cartridges that hold dirt and debris. These cartridges require no additional media to filter, as a D.E. filter does, and cleaning them is quite simple. Cartridge filters do not backwash as a D.E. filter does. To clean a cartridge filter when it gets filled with dirt and debris and the filter pressure rises you merely have to remove the cartridges from inside the filter and hose them off with a garden hose. This cleans the dirt and debris from the cartridge making it clean and ready for use again. Cartridge Filter Note Cartridge filters do not filter as well as D.E. filters do. This will be most evident at night time when the pool light is on you will often see more fine filaments of debris floating in the light. A cartridge filter will have a rubber tank o-ring, just as a D.E. filter does and such should be lubricated just as with a D.E. filter. The disassembly and reassembly of a cartridge filter will be similar to that of a D.E. filter with the exception of having a large or several smaller cartridges inside instead of filter grids. Cartridge filters do not filter as thoroughly as D.E filters do, being that Diatomaceous Earth is a much finer filter media; however they are a commonly found and work well. After cleaning and reassembling a cartridge filter turn the pump on and make sure there are no leaks. When cleaning the cartridges of a cartridge filter be sure to hose them off in a place that will not make a mess of the customer’s yard. They generally do not appreciate the mess and making a mess of the customer’s yard would be seen as unprofessional and careless. Sand Filters: Sand filters are rarely used for swimming pools in southern California and are more commonly seen in other states. Sand filters, in function work similarly to D.E. filters in that they can be backwashed, have backwash valves and should be backwashed about once per month. Sand filters are mostly if not always plastic or fiberglass. When you are backwashing a sand filter, allow it to backwash for a couple of minutes, more so than you would with a D.E. filter. There is no powder or other filter media to add to a sand filter. Sand Filter Note Sand Filters are commonly used for ponds or in areas where Diatomaceous Earth back washing is not allowed. Sand filters tend to filter better than Cartridge Filters but not quite as well as D.E. filters. Inside a sand filter is a large compartment that holds fine sand. The water is pushed through this sand and the clean water is returned to the pool. The sand in this inner compartment collects the dirt and debris and serves as the filter media. Backwashing a sand filter once a month will blow much of this dirt and debris out of the sand and send it to the sewer line, just as it would with a D.E. filter. Once every few years the sand inside a sand filter should be removed and replaced with new sand. Check the manufacturer’s information regarding what sort of sand and what quantity of sand to add. 63 Things to Remember About Section 13 There are three types of filters commonly in use for swimming pools and spas. They are Diatomaceous Earth Filters, Cartridge Filters and Sand Filters. Diatomaceous Earth filters are made up of a series of fine mesh grids with a plastic structure that are contained within a plastic, fiberglass or metal tank. These fine mesh grids hold on to the Diatomaceous Earth (D.E.) or also called filter powder which coats the grids. The D.E. powder is what actually does the filtering while the grid’s job is to hold the powder in place. A D.E. filter can be back washed which flushes out most of (but not all) of the current D.E. powder, dirt and debris inside the filter so that you can replenish it with fresh new Diatomaceous Earth. This process reduces the filter pressure, cleans out much of the dirt and debris and allows for strong suction and return of the water. Diatomaceous Earth is a carcinogen and can cause cancer. It is so fine that if breathed in it will remain in your lungs. Cartridge Filters are similar in shape and size to a D.E. filter and contain a simple fine mesh material or cloth like material cartridge inside the filter tank which collects the dirt and debris. Cartridge filters do not require any special powder as the cartridge itself does the filtering. Cartridge filters do not filter out debris quite as well as a D.E. filter but work well and will still be commonly found in the field. When cleaning the cartridges of a cartridge filter, or any filter for that matter, be sure to hose them off in a place that will not make a mess of the customer’s yard. They generally do not appreciate the mess and making a mess of the customer’s yard would be seen as unprofessional and careless. Sand filters are rarely used for swimming pools in southern California and are more commonly seen in other states. Sand filters, in function work similarly to D.E. filters in that they can be backwashed, have backwash valves and should be backwashed about once per month. Sand filters are mostly if not always plastic or fiberglass. When you are backwashing a sand filter, allow it to backwash for a couple of minutes, more so than you would with a D.E. filter. There is no powder or other filter media to add to a sand filter. Once every few years the sand inside a sand filter should be removed and replaced with new sand. Check the manufacturer’s information regarding what sort of sand and 64 what quantity of sand to add. 14. Different Types of Pool and Spa Surfaces There are many different types of swimming pool and spa surfaces. Some of these surfaces are durable and resistant to wear and staining, while others are more sensitive to adverse conditions and heavy use. Here we will discuss the various types of surfaces used for swimming pools, difference in their care and chemistry and also the many types of staining you may see on these surfaces and their causes. We will go over Plaster, Pebble Tech, 3M Color Quartz, Fiberglass, Acrylic, Vinyl Liners and Painted pool surfaces. Plaster: Plaster is the most common and widely used surface among swimming pools. Plaster can come in white, grey and black though other custom colors can also be formulated. Plaster is very delicate at start-up when a pool is first surfaced. You must NEVER vacuum a plaster pool for at least the first 5 weeks with brand new plaster as the rollers on the vacuum will leave permanent marks on the soft new plaster. The surface of the plaster takes about one month to cure, making it durable enough to vacuum without leaving marks. When doing a start-up service on a new plaster pool use a “Brush-Vac” type of vacuum head to vacuum the pool. A brush-vac has bristles, much like a wall brush, instead of rollers so it will not leave roller marks on the delicate plaster. The chemicals on a chemical start-up for new plaster must be added very slowly; spread out over a month else any harsh changes in the chemistry may cause staining, discoloration and mottling to the new plaster. It is best to have a professional do a chemical start-up on brand new plaster until you have been trained properly in doing so. Usually, each plasterer will have different specific ways in which they want the chemicals started up on their plaster. White Plaster: White plaster is the most common swimming pool surface in the world and is also the easiest surface to see staining. Being that calcium is the most abundant mineral in the water it is good to have white plaster, as when the calcium falls out of circulation, which it does frequently, you can not see it as it is as white as the plaster. Calcium will provide an anchor for other minerals and metals to stick to when they fall out of circulation. Often, other stains that you see on a white plaster surface are actually stained on top of the calcium fallout. An acid wash will remove a fine layer off the top of the plaster and much of the surface staining along with it but not always all of it. A plaster surface can only be acid washed a few times before becoming too thin and porous revealing the gunite structure below the plaster. Caution! Never use Trichlor Granular (Algae Ban) on a dark plaster surface as the acid in the Trichlor will cause severe burning and staining to the surface. Grey and Black Plaster: Grey and black plaster are the same as white plaster with the exception of staining. On grey and black plaster surfaces you will see calcium staining much more than on a white plaster surface. This calcium staining will create a white and grey mottling effect over the surface and can not be acid washed off. This calcium staining occurs fairly quickly, starting over the first few months and grows substantially worse over time. Often a plasterer will not tell a customer about this mottling effect and most customers are disappointed from the effects of this staining. Draining and refilling grey and black plaster pools more frequently will help to keep 65 the Calcium Hardness levels down and reduce the risks of staining, however to my experience it is always just a matter of time before the surface becomes mottled with calcium stains. Pebble Tech: A Pebble Tech surface is a series of tiny pebbles of a variety of colors all blended to make for a single shade of color. Pebble Tech surfaces come in a wide variety of colors and make for a very natural, beach like appearance. A Pebble Tech surface is much more durable than plaster and requires no brush-vacing at start-up. When adding chemicals at the start-up of a new Pebble Tech pool it Did you know? is still a good idea to take it slow and spend a month getting all of the chemical levels up to their desired Pebble Tech is composed of very levels as adding too many chemicals all at one time is small, rounded pebbles of various never a good idea. Pebble Tech should never be taken all the way up to the surface as the calcium staining usually seen along the water line and at the spa or other spill ways is very difficult to clean off of Pebble Tech. At the water line the surface should always be a cleanable surface such as tile or artificial painted rock that can be repainted. colors mixed to form a particular and even sheen of color and is then mixed with plaster. Chemically Pebble Tech pools behave much the way a plaster pool would. Pebble Tech pools, usually due to their darker colors tend to absorb heat better and stay warmer. Most surface staining is lost on a Pebble Tech surface, meaning it is so well camouflaged into the pebbles it is barely noticeable, if seen at all with the naked eye. Severe staining can still be seen, however severe staining should not occur if the water is well maintained and properly balanced. The surface of Pebble Tech can also be acid washed, just like plaster should any noticeable staining occur. Black or the darkest grey shades of Pebble Tech are not recommended as being that calcium staining is white it will be noticeable on the surface and even after an acid wash will likely return over a period of a few months. Pebble Sheen is a Pebble Tech type of surface made with smaller, finer pebbles to produce a smoother finish. This smoother surface is less dynamic in appearance compared to the more vibrant and noticeable pebbles of Pebble Tech. From personal experience I have found a Pebble Tech surface to be very forgiving to surface staining, very esthetically pleasing, more difficult to notice dirt and debris as well as quite durable. I highly recommend it. 3M Color Quartz: 3M Color Quartz, also called 3M Quartzite has the smooth, even finish of plaster with the durability of Pebble Tech. To the touch it feels, not as clay-like or smooth as plaster but more of a smooth concrete, lightly textured feel. 3M Color Quartz has very tiny crystals mixed into it giving it a very vibrant and unique look and shines brilliantly at night from the pool light. It can be mixed into a wide variety of colors, although white with blue crystals seems to be most common. 66 3M Color Quartz is not as delicate as plaster and does not have to be brush-vaced at start-up. It can be vacuumed up immediately with a regular vacuum. Apply the start-up chemicals slowly as with any other pool, taking several weeks to slowly achieve the desired balanced water chemistry. 3M Color Quartz is fairly stain resistant, though it can still be stained and the stains will be noticed more or less depending upon the color chosen. For white 3M Color Quartz, just as with white plaster, calcium fallout will not be noticed, however other staining will stand out more. For any darker 3M Color Quartz surface calcium fallout, being white will be much more noticeable. 3M Color Quartz can be acid washed to clean off a fine layer off the top just as plaster and Pebble Tech can. It is a very tough and durable surface, resistant to staining and easy to work with. Fiberglass and Acrylic: Fiberglass pools and Acrylic spas have a very hard plastic, smooth and polished feel to the touch. While fiberglass is not as common as plaster surfaces they will be encountered now and again in the field. Caution! Above ground Acrylic spas are fairly common and are Be VERY careful when adding Muriatic usually covered. A fiberglass coating over an existing Acid or even 3 inch Trichlor tablets to a plaster surface is often sold as a less expensive Fiberglass pool. Fiberglass does not have alternative to re-plastering a pool and such resurfaced alkaline material to give off on a regular pools will be more commonly found in lower income basis the way that plaster does. Even a small areas, although they can be encountered just about amount of Muriatic Acid or a few Trichlor anywhere. Acrylic spas are mentioned here as Acrylic tablets can lower the pH balance drastically. is a very similar surface and is treated similarly. Be prepared to have extra Soda Ash and sodium bicarbonate handy when you are There are many pros and cons to having a fiberglass servicing Fiberglass pools. surface for a swimming pool. Actually, there are only two advantages to having a fiberglass surface worth mentioning. Fiberglass surfaces are less expensive than re-plastering a pool and fiberglass is also very resistant to staining. There are many more cons to a fiberglass surface. They are: A fiberglass surface is very polished, even reflective; much more so than plaster porous type surfaces. It is much like the difference between flat primer paint and gloss paint. This polished surface reflects the Sun’s harmful Ultra Violet rays allowing these rays to bounce off the surface and pass through the chlorine in the water more often. This means that a fiberglass surface creates a higher chlorine demand for the water as the chlorine is damaged and degraded more rapidly than in a plaster or other non-reflective surface. Expect to use more chlorine in these types of pools, even when the pool is not in use and especially in direct sunlight. The smooth, polished surface of fiberglass can be a bit more slippery for swimmers and also for dirt and debris at the bottom. Rather than clinging more easily to the surface, often dirt and debris will float around and scatter more, making it more difficult to vacuum effectively. 67 A fiberglass pool is VERY sensitive to acid. The fiberglass surface is non-alkaline, meaning it does not give off any alkalinity as plaster, Pebble Tech or 3M Color Quartz surfaces do. When adding Muriatic Acid to lower the pH Balance or any chemical that is acidic, such as 3 inch Trichlor Tablets always be VERY mindful of how much acid you are adding. A fiberglass surfaced pool can turn acidic very quickly. When adding Muriatic Acid to a fiberglass pool, as a general rule add only half the amount that you would in any other pool, just to be on the safe side, but always test for the Total Alkalinity to make sure there is plenty of alkaline buffer in the water. In a fiberglass surfaced pool it might even be a good idea to maintain the Total Alkalinity level a little higher than normal to make up for the water’s extreme sensitivity to acid. I personally advise using Liquid Chlorine as an oxidizer and sanitizer for a fiberglass surfaced pool as liquid chlorine has a high pH Balance and you will find yourself more often than not trying to raise the pH of a fiberglass pool. As a fiberglass surface deteriorates over time it will crack, chip and pieces of it, like old, brittle, dried out broken plastic will start to come free of the surface leaving behind the concrete or plaster under the surface. NEVER use a steel wire wall brush on a fiberglass surface as it will scratch and damage it. Even in the even of Black (blue-green) Algae a steel wire wall brush should never be used. I do not recommend the use of fiberglass as a swimming pool surface, but there are many of them in use. They are often sold as a cheap alternative to re-plastering for customers who are on a low budget. Vinyl Liners: Vinyl liners are more commonly found on above ground pools. Many of us recall knowing someone who had an above ground “Dough Boy” pool in their backyard, or perhaps they saw a “blooper video” on television where an above ground vinyl lined pool was torn or broken and the pool spilled out all over the yard all at once. Some vinyl liners are placed on an in-ground pool over a concrete structure. Either way the principles of a vinyl lined pool remain the same. Vinyl is a thick plastic like material that is held in place by a metal frame or perhaps it is lining a concrete structure. A darker color of vinyl is also commonly used as the water-proof liner for fish ponds, man-made streams and other natural backyard water features. More than likely you will never come across a vinyl lined swimming pool for service, but on the off chance that you do here is some information about them. When servicing a vinyl lined pool treat it chemically as you would a fiberglass pool, being VERY careful when adding acid or any acidic chemical. NEVER use a steel wire wall brush and when using a regular wall brush be very gentle. The vinyl liner is obviously very susceptible to tears so always use caution with any sharp or pointed tools around them. It is not the kind of surface that you accidentally want to gouge your metal pole into. While I do believe they make patch kits for damaged vinyl liners, when damaged it is likely best to replace the liner completely. Personally, as a service technician I avoid these types of pools entirely as they are just not very profitable swimming pools to service compared to the risk of damage. It only takes one tear in an above ground vinyl lined pool to flood out a customer’s house and make quite the claim on your insurance. 68 Painted Pools: Painting a plaster surface is one of those things that “seemed like a good idea at the time” but ends up causing more problems than it solves. Every now and again a pool owner will, instead of re-plastering or re-surfacing their pool, decide to paint over the surface. Some do a very nice job of it, cleaning the surface first, giving it a good primer coating and laying a couple good coats of paint on to make sure it looks nice. Sometimes it doesn’t even appear to be all that bad if the pool owner takes the time to do a good and thorough job of it, but don’t be fooled. Painting a pool is never a good idea. You will come across painted surfaces more often in lower income areas as paint is the lowest cost alternative to doing any other kind of re-surfacing to a swimming pool. Water’s nature is to penetrate though any other material to reach its lowest point of gravity. It penetrates through paint quite effectively. Paint will regularly break down, clouding up the water and will show any defects or thin areas in the paint job rather quickly. This continuously breaking down paint will clog the filter as it is vacuumed up or circulated through the system. Depending on what kind of paint was used, whether it was a gloss or flat kind of paint for example, the chlorine demand on a painted surface can vary. Never use a steel wire wall brush on a painted surface as it will scrape away the paint. Painted surfaces can sometimes be sensitive to acid, just as a fiberglass pool, depending on how well the plaster was sealed before it was painted. Each painted pool’s chemical treatment, because of these many factors will vary on a pool by pool basis. In general, even a good paint job does not remain appealing for long. Again, painting a swimming pools surface is never a good idea. Other Notes on Swimming Pool Surfaces: There are some other surfaces that I have not covered here such as a surface made up mostly or entirely of tile and there are always new surface materials entering into the industry. When you encounter tile surfaces treat them as you would a fiberglass surface. Always be weary when adding Muriatic Acid or any other acidic chemical and NEVER use a steel wire wall brush on them. When in doubt, contact the manufacturer for recommended chemical levels and services on these types of surfaces. Your local swimming pool equipment supplier will almost always have access to phone numbers for manufacturers or other contractors with experience on such surfaces. Keep in mind that the more reflective a surface is during times of direct sunlight, the faster the chlorine in the water will degrade and burn off, creating a high chlorine demand even if the pool is not in use. Things to Remember About Section 14 There are many different types of swimming pool and spa surfaces. Some of these surfaces are durable and resistant to wear and staining, while others are more sensitive to adverse conditions and heavy use. Plaster is the most common and widely used surface among swimming pools. Plaster can come in white, grey and black though other custom colors can also be formulated. Plaster is very delicate at start-up when a pool is first surfaced. You must NEVER vacuum a plaster pool for at least the first 5 weeks with brand new plaster as the rollers on the vacuum will leave permanent marks on the soft new plaster. 69 When doing a start-up service on a new plaster pool use a “Brush-Vac” type of vacuum head to vacuum the pool. The chemicals on a chemical start-up for new plaster must be added very slowly; spaced out over a month to avoid staining, discoloration and mottling to the new plaster. Never use Trichlor Granular (Algae Ban) on a dark plaster surface as the acid in the Trichlor will cause severe burning and staining to the surface. A Pebble Tech surface is a series of tiny pebbles of a variety of colors all blended to make for a single shade of color and a very natural, beach like appearance. A Pebble Tech surface is much more durable than plaster and requires no brush-vacing at start-up. Pebble Tech should never be taken all the way up to the surface as the calcium staining usually seen along the water line and at the spa or other spill ways is very difficult to clean off of Pebble Tech. Black or the darkest grey shades of Pebble Tech are not recommended as being that calcium staining is white it will be noticeable on the surface and even after an acid wash will likely return over a period of a few months. 3M Color Quartz, also called 3M Quartzite has the smooth, even finish of plaster with the durability of Pebble Tech. To the touch it feels, not as clay-like or smooth as plaster but more of a smooth concrete, lightly textured feel. 3M Color Quartz has very tiny crystals mixed into it giving it a very vibrant and unique look and shines brilliantly at night in the pool light. 3M Color Quartz is not as delicate as plaster and does not have to be brush-vaced at start-up. It can be vacuumed up immediately with a regular vacuum. Apply the start-up chemicals slowly as with any other pool, taking several weeks to slowly achieve the desired balanced water chemistry. Fiberglass pools have a very hard plastic, smooth and polished feel to the touch. Be VERY careful when adding Muriatic Acid or even 3 inch Trichlor tablets to a Fiberglass pool. Fiberglass does not have alkaline material to give off on the way that plaster does. Even a small amount of Muriatic Acid or a few Trichlor tablets can lower the pH balance drastically. Fiberglass surfaces are less expensive than re-plastering a pool and fiberglass is also very resistant to staining. Fiberglass is more reflective to the Sun’s U.V. rays. This reflective property means that the chlorine in the water becomes bombarded more so and destroyed more quickly than in a regular plaster pool. NEVER use a steel wire wall brush on a fiberglass surface as it will scratch and damage it. Even in the even of Black (blue-green) Algae a steel wire wall brush should never be used. Vinyl liners are commonly found on above ground pools or or vinyl is placed over an inground concrete base. When servicing a vinyl lined pool treat it chemically as you would a fiberglass pool, being VERY careful when adding acid or any acidic chemical. NEVER use a steel wire wall brush and when using a regular wall brush be very gentle. The vinyl liner is obviously very susceptible to tears so always use caution with any sharp or pointed tools around them. Never use a steel wire wall brush on a painted surface as it will scrape away the paint. 70 15. Types of Surface Staining “Surface stains are the result of algae, deposited heavy metals, leached tannin from tree debris or fungus growing on the reverse side of the liner in a vinyl pool. This section deals with heavy metals. The main culprits are copper and iron, and sometimes manganese; problems from cobalt, silver, and other metals are much less common. Colored water caused by dissolved metals can be the first hint of trouble: translucent green, blue/green, red, brown, or black/purple. Green color is produced by either copper or iron, blue/green by copper, red and brown by iron, and black/purple by manganese. Colored water usually appears after a pool is filled or after an oxidizer such as chlorine has been applied. Oxidation will cause metals to fall out of solution and stain the shell surface. Where do these metals come from? 1. Water used to fill the vessel, particularly well water: copper is rarely found in well water, but iron and manganese are. Iron will precipitate as a brown deposit, manganese as a black-grey. 2. Unbalanced water allowed to corrode metal equipment or piping: corrosive water will dissolve copper piping, heat exchangers, steel filters, and other metal fittings and equipment. Copper stains are blue-green but can turn grey-to-black as copper oxide forms in the presence of chlorine. 3. Improperly maintained ionization systems 4. Improperly dosed copper-based treatment products primarily algaecides: can result in a bluing effect on the pool walls that could lead to a grey-black stain. 5. Foreign metal objects left in the water Sequestering and chelating (“key-late-ing”) treatments bond with any dissolved metal in the water and keep it from precipitating as a stain. These chemical compounds are also referred to as stain and scale inhibitors/preventors, metal suspenders, and metal removers. Some are strong enough to pull metal deposits off the surface and back into solution, thereby removing the stain. Draining a badly discolored plaster pool and then acid washing the walls with diluted Muriatic acid will remove a thin layer of the plaster, taking many of the stains with it.” – Taylor Test Kit Pool & Spa Water Chemistry Guide Conditioner (Cyanuric Acid) can also fall out of solution past 100 ppm (parts per million) causing varying degrees of purple staining. This staining will appear to be anything from a light purple dust coat over the surface to a dark dust coat and purple vertical lines in the walls. 71 Things to Remember About Section 15 Iron staining is usually brown to a black-grey. Copper staining is turquoise to dark blue and can turn black when oxidized or otherwise then called copper oxide. Plants that contain iron from the soil can also cause iron staining from reddish brown to black. Conditioner over 100 ppm can fall out of circulation and can cause light purple staining. Sequestering and chelating (“key-late-ing”) treatments bond with any dissolved metal in the water and keep it from precipitating as a stain. 16. A Typical Service Stop This is what a typical stop on a regular day could be like, just to give you a hypothetical example. This stop should take about 20 to 25 minutes. 1. I start by parking my truck, grab a door hanger from my glove compartment and slip it into the door jamb on my customer’s front door to let them know that I have been there that day for service. 2. I don’t use an equipment cart, though it is common for other pool technicians to use them, so I grab my extendable pole, my tile brush with a little tile soap on it, and my wall brush and test kit all held inside my regular net, using my net to hold everything. 3. Entering the backyard, I’ll make sure the gate is closed securely behind me and walk to the swimming pool area. I put all my equipment down by the skimmer. There might be a dog or two in the yard so I’ll give ‘em a quick pet, throw ‘em a tennis ball then it’s time to work. 4. I notice that the pool’s water level is low so I be sure to mark on the door hanger for the customer to add water. Service Note During the swim season you will likely find many pool toys in and around the pool. It is always a good service courtesy to remove the toys from the pool so that they do not lure small children toward the water and to generally make for a more tidy pool area. 5. Very quickly I’ll test the water’s Free and Available Chlorine , pH Balance and Total Alkalinity. About once Caution! a month I’ll test the water’s Conditioner level, Do not add water to the pool. Most unless I am having problems keeping chlorine pool tech liability insurance claims come in the water, in which case I will test for from the service tech forgetting to turn Conditioner then and there. I test for the off the water. It is not your water’s Calcium Hardness about once every 6 responsibility to add water to the pool. months, unless it is my first time there or a Alert the home owner that their water brand new chemical start-up and keep track level is low and if the low water level of when it is getting to 500 ppm (parts per poses a danger to the pool than turn the million) to alert the customer that it is time to time clock off, however do not add drain and refill the pool. water. If low water becomes a regular problem than report this issue to your service manager and suggest installing an automatic water timer such as a “sprinkler thinker” or other brand. 72 6. I go out to my truck and grab whatever chemicals I need to rebalance the water. This will typically be a pound or two of granular chlorine or a couple gallons of chlorine depending on my chemical regiment at the time which I’ll carry in an orange D.E. scoop, a half gallon or so of acid and maybe a few 3 inch chlorine tablets for the floater or chlorinator. If it is Spring or Summer I might also add a couple ounces of Sodium Bromine. 7. I return to the pool, again making sure the gates behind me are closed and secure and head back to the pool to add the chemicals. 8. I’ll dunk the gallon container of acid and chlorine if I am using liquid in the pool and then pour a half gallon of it in the pool, walking it around as I pour. I’ll then dunk the gallon container again to make sure there are no acid drips on it and set it down on the deck, usually on the skimmer lid just to be safe. 9. If I am using quick dissolving granular chlorine I’ll add it directly to the pool, knowing it will be dissolved by the time it hits the plaster. I’ll put the 3 inch chlorine tablets in the floater, which should be tied off at the pool’s edge somewhere. 10. Now that the chemicals have been added, I can start on the manual labor. I grab the tile brush with a little tile soap and very quickly brush around the tile, walking and scrubbing very briskly. I’ll give an extra scrub along the spillway of the spa, being there will usually be extra calcium build up there. Caution! If a dog is aggressive and will not allow you access to the pool area than do not attempt to service the account. Leave a door hanger and a note on their door and contact the customer to arrange a service day and time that the dog can be put in the house or locked away. Do not risk injury. 11. I’ll open the skimmer and pour the leaves and debris in the skimmer basket into my net. I will also go over to the pump basket and inspect it for debris. If there is any debris inside the pump basket, anything substantial anyhow, I will quickly open it up and dump its contents into my net and close the pump up. While I am there by the equipment I will turn the pump on to regain prime to the pump, since I just opened it. 12. If my net is getting full from the debris from the skimmer and pump baskets than I will quickly empty it into a near by trash can. 13. I will net out any surface debris, leaves and such on the surface of the water and then quickly net the floor of the pool for any large leaves or debris. 14. After netting out the larger debris in the pool I will again empty my net into the trash can. 15. If while I was netting the floor of the pool there was dust than I’ll likely have to vacuum the pool. Usually, if there is no dust than I wont vacuum but realistically I would say that I have to vacuum about every other stop, unless it has a floor cleaner. Some technicians “claim” to vacuum every time but come‘on…let’s be realistic here. I’ve only got 20 minutes. Some days vacuuming just isn’t gonna happen. 73 16. I bring my net, test kit, D.E. scoop that I held chlorine and tabs in, half gallon of acid and chlorine bottles and my tile brush all back to the truck. I leave only my wall brush and pole behind in the yard. While I am at the truck I grab my vacuum and head back to the pool, again making sure the gate is closed and secure so the dogs don’t get out and kids don’t get in. 17. I attach the pole to the vacuum and put the pole and vacuum in the water. I’ll proceed to feed my hose into the water, unwinding it with one hand and feeding it into the water with the other to fill my hose with water. I don’t want all the air in my vacuum hose to go to the pump as the pump will lose prime and I’ll have to wait a while to get all the air from my hose through the system. 18. Once my vacuum hose is full of water I will feed it into the skimmer and plug it into the suction line inside. I also want to make sure that the valves are set correctly to get the optimum suction. 19. Vacuuming is the slowest part of the job. It will usually consume about half of the time you spend in the backyard so be sure and do everything else quickly. I begin thoroughly vacuuming the surface of the pool; usually the bottom and a little up the walls, going back and forth covering the entire pool floor. Being a multi-tasker this is usually when I’ll get some daily phone calls out of the way as I have a little time to kill being that vacuuming can be a slow process. An “I-pod” or other music devise is handy at times like this as well. 20. Once I am done vacuuming the pool, and spa if they have one, than I’ll unplug my vacuum hose from the skimmer, pull the vacuum and pole out of the water, unattach the pole, laying it to the side (watch out for windows behind you, or anything else breakable for that matter), close up the skimmer and begin winding up my vacuum hose. I usually keep my vacuum hose contained using a bungee cord. 21. When I’m done wrapping up my vacuum hose, I’ll attach the wall brush to my pole and quickly brush down any dust from the pool walls. 22. Before I leave I turn the pool pump off and fill out the service log by the time clock writing in the date, time, chemical readings and such. I will also take a moment to inspect the equipment making sure there are no leaks or problems. 23. Give the dogs a farewell pet, toss ’em the tennis ball once more and it’s back to the truck, making sure the gate is securely closed. Service is done. Figure on 20 to 25 minutes depending on the size of the pool. 74 Things to Remember About Section 16 Always! Always! Always! Make sure the gates are securely closed, even if there are no dogs in the yard. Always communicate with the customer whether it be with a door hanger, sign-in log or otherwise. No-shows or “assumed” no-shows from poor service are one of the most common reasons for a customer to cancel service. Never leave a mess in the customer’s back yard. Do not leave diatomaceous Earth around the skimmer, mess from a filter clean or drips from liquid chlorine or Muriatic Acid. Always test for chemistry balance carefully and thoroughly. Never take shortcuts when it comes to chemistry. 17. Common Problems Encountered in the Field Many things can happen while servicing a swimming pool in the field. Usually only experience will teach you what to do. Here are some commonly found problems and their solutions to help speed up your learning curve and hopefully relieve some frustrations. There are also some suggestions here to help make your swimming pool service experience a little easier. These problems, solutions and suggestions are in no particular order. Problem: The suction is very weak when I try to vacuum the pool. Solution: 1. Is the filter dirty? Try backwashing the filter. 2. Is the pump basket full? Try emptying the pump basket. 3. Is air returning back into the pool through the return lines? There could be a suction side leak in the plumbing somewhere causing the pump to suck air through the leak rather than water, thus losing your suction. 4. Is the suction side valve adjusted properly? It could be set to suck from the main drain or a floor cleaner. 5. Is it a “one hole” skimmer with an adjustable “j-valve” diverter? You could have to turn the diverter inside the skimmer to get optimal suction. 6. Are there 2 skimmers? Some systems are plumbed in with 2 skimmers and when resistance is created at one skimmer, water will follow the path of least resistance and go to the other skimmer. Plugging the other skimmer with a tennis ball, if it doesn’t otherwise have a valve, will force suction to the skimmer that you are vacuuming from. 7. Is there an obstruction in your vacuum head? Sometimes a rock or stick will plug up the bottom of the vacuum head and stop suction. Problem: There is a white line of calcium along the tile at the water line and also at the spillway of the spa. Solution: 75 1. This calcium is left behind from evaporation. Only the H2O evaporates, leaving other minerals and metals in the water behind as deposits. Calcium is the most abundant mineral in the water. While you can clean off this calcium with a wide variety of scale removers, it will continuously return. Try running the pump at night time when there is less evaporation to reduce the scale and calcium deposits. Also check your pH Balance, Total Alkalinity and Calcium Hardness to make sure they are all balanced. Problem: There is plenty of water in the pool, but the pump seems to be sucking air and cavitating. Solution: 1. Check the skimmer opening for an obstruction such as a pool toy. Also check the “weir blade” (plastic floating door inside the skimmer) to make sure it is not stuck. 2. If there is a floor cleaner, check the hose to make sure there are no cracks in it where it could be sucking in air. Also check the floor cleaner suction valve to make sure it is not turned on too strong. 3. Check the plumbing for a suction side leak such as where the plumbing is attached to the pump or the valves on the suction side of the plumbing. Inside the valves are usually small rubber o-rings and when they corrode they can crack and cause leaks. Problem: There is air in the pump basket and I can not get the pump to gain prime. Solution: 1. Open the pump basket and fill it with water, then close it up and turn the pump on. This water in the pump basket may be enough to help the pump gain prime. 2. If the spa is raised above the pool, try turning the suction side valve over to spa suction. Water will try to get to its lowest point of gravity and may be easier to flow from the spa than from the pool. Once suction and prime are established, return the valve back over to pool suction as to not drain down the spa. 3. Once prime is established, bleed the air out of the filter by opening up the air bleeder valve on top of the filter. If there is air inside the filter it will be compressed. If the pump is turned off than that compressed air will suddenly decompress, sending water rushing back to the pool backwards through the system and will empty the pump basket. 4. Consider plumbing in a check valve on the suction side of the plumbing before the pump but after the suction side valves. This will help keep water in the pump and will also prevent water from draining out of the filter in the case of compressed air. Problem: Some large leaves and pods or berries from a tree have left a reddish-brown staining on the surface of the pool. The stains do not brush off. Solution: 1. This staining is usually temporary, only lasting a week or two and is caused by the oxidation and decomposition of the leaves and berries. The staining is possibly caused by the color of the leaves and berries, much like a dye and maintaining proper water chemistry 76 should clear up these stains over time. If the stain remains longer than a couple of weeks it could also be rust staining caused by iron absorbed by the leaves from the soil. Problem: The rocks or tile along the planter by the pool are turning white with calcium, but there are no water features or water spilling there. Solution: 1. This water and calcium are not coming from the pool, but is coming from the planter. If the planter was not properly sealed from the back where the soil and moisture are than water from the planter will seep through the rocks and tile, collecting calcium along the way and depositing the calcium on the rock or tile surface where the water evaporates. Suggestion: 1. When adding water to the pool through the fill line or a garden hose ALWAYS leave your keys by the handle of the fill line or hose. This way you will always remember to turn off the water before you leave and not overfill the pool, as you can not leave the account without your keys. Suggestion: 1. Make sure there is a functioning “weir blade” inside each skimmer. This will help the skimmer to collect as much debris from the surface of the water as possible, making your job of cleaning the debris easier. 2. When installing a “weir blade” to the skimmer always put in a size just slightly smaller than the size of the skimmer opening. For example if the skimmer opening measures 8 inches use a 7 13/16 inch “weir blade”. This will make sure the “weir blade” can swing freely without getting stuck on the sides of the skimmer from fitting too tight. 3. When using an after market spring loaded “weir blade” you can make the spring bar that holds the “weir blade” in place fit a bit more tightly by placing a very small rock inside the tube. This will in effect lengthen the spring bar just slightly allowing for a tighter, more secure fit. Problem: The filter pressure is high but the filter is clean. Solution: 1. Some plumbing systems can cause additional filter pressure. A pop-up head floor cleaning system has very small jets and will cause additional back pressure on the filter. A solar heating system on the roof of the house will also cause additional filter pressure. If the spa is way above the pool’s plumbing it can cause additional filter pressure. The pump in trying to force the water either up hill or through very small return lines will create back pressure on the filter, meaning that the water has no other place to go and its additional pressure is built up in the filter. Suggestion: 1. For pools with large amounts of very fine or small debris floating on the surface you can squirt a stream of “Liquid Skimmer”, a mild bottled liquid soap type product down the center of the pool. This soap type product will cause the debris to move to the sides of the pool, clumping up to make it fast and much easier to net out, saving you time and energy. 77 Suggestion: 1. Avoid walking on a tile surface whenever possible as the tile can be extremely slippery. If you do have to walk on a tile surface use extreme caution, walking very slowly and flat footed. A slip on the tile can cause very serious injuries. Suggestion: 1. When using liquid chemicals such as Muriatic Acid and Liquid Chlorine always be sure to dunk the bottle into the pool water both before pouring its contents into the pool water and also afterwards. This will help to rinse off any drips that may run along the side of the bottle and cause staining should you set the bottle down on the deck. Suggestion: 1. It is recommended to have extra pump baskets and skimmer baskets for your most common pumps and filters on hand in your vehicle. During the wind season you will find many of these baskets will split and break, being over filled with leaves and debris. 2. It is recommended to have extra 3 inch chlorine tablet floaters in your vehicle. You never know when an old one in a pool will corrode and fall apart and it is always handy to have a few spares handy, particularly in the summer when 3 inch chlorine tablets are more in use. 3. Keep a spool of string handy to tie off any 3 inch chlorine tablet floaters and prevent them from floating over the top step and causing damage to the surface. Suggestion: 1. Get in the habit of securely closing every door and gate you go through, especially the pool gates. This will help to avoid any unsupervised children accessing the swimming pool area and will also help to avoid losing any pets should they escape out the back gate. It doesn’t take much for that $50.00 mixed breed dog from the pound to suddenly, in court become a $5,000.00 family heirloom. Suggestion: 1. When you start service try to keep all of your equipment together in the same area. This will help to prevent forgetting any equipment in the backyard when you leave for your next service stop. Suggestion: 1. Particularly in the summer time when swimming pools are used more often, check for the water’s chemical balance and add chemicals first, that way the chemicals are well circulated by the time you finish service and awaiting swimmers can jump back into the pool. 2. Don’t forget to have swimmers rinse their faces off with water before entering the pool. This will rinse off most of the organic material and swimmer waste such as sweat, dead skin cells and dirt and make the water more comfortable for the swimmer, avoiding any foul smelling and uncomfortable and irritating chloramines. Suggestion: 1. When servicing a swimming pool, regardless of what season or time of year, always be mindful of the time. A service stop should only take about 20 minutes. If you spend too much time at a service stop you will run out of time to finish everything by the end of the 78 day, particularly in the winter time when it is dark by 5pm and the pools are weather damaged. 2. The regular monthly bill you charge to your customers only covers a basic, standard 20 minute service each week. If a swimming pool has severe amounts of debris from wind conditions, rain or local fires or other unforeseen conditions than this “extra” service should be requested at an additional charge as it is not part of their regular monthly bill. Make sure your customers are aware of this. Suggestion: 1. If the landscapers or gardeners blow or knock large amounts of debris into the swimming pool, causing you extra work be sure to alert the customer of this condition and that to clean it requires additional time and a service charge if such applies. You have enough work to do without doing the landscapers job as well. 18. Examples of Chemicals Added at Typical Service Stops Here I will give examples of various water conditions over several different types and sizes of swimming pools at different times of year to give you a rough gauge on what to add and why or at least how I add chemicals in the field. There will be examples, conditions, chemicals added or service given and why. Example #1 Conditions: It is summer time, very hot and the pool is getting a lot of use. The pool is a larger than average, 25,000 gallon white plaster pool and spa. There was 0 Free and Available Chlorine reading, the Ph Balance is 7.6 and the Total Alkalinity is 110 ppm (parts per million). The water is a little cloudy and there is a small amount of Mustard (yellow) Algae. Actions: Hmmm….not good. This pool needs attention. Let’s handle it. First off, why is the algae growing, why is the pool cloudy and where did the chlorine go? I checked the Conditioner level, to make sure my chlorine was being well protected and got a reading of 70. 70 just isn’t good enough. The heavy use from the kids must have splashed out a lot of Conditioner, so I’ll definitely be adding that. I turned on the pump and checked the filter pressure. The pressure was high for that pool at about 26 psi (pounds per square inch). I found the PoolRX I had in the pump basket tossed in the bushes. Also not a good sign. If I had to guess, I would say they had a pool party recently, or some extremely heavy use, which is why the water is cloudy. There is plenty of organic material and swimmer waste in the water from all the usage and no sanitizer or oxidizer to clean it up. I would backwash the filter to lower the filter pressure, giving the pool water better circulation and filtration. The pH Balance is rather high so I would add a full gallon of Muriatic Acid, knowing that the Total Alkalinity is high enough to absorb it easily, and it is a larger than average size pool. This will lower the pH Balance so that when I add chlorine to the pool water it will be aggressive and more so in its killing form. I would add about 5 lbs. of Dichlor granular (or 5 gallons of liquid) chlorine. This might seem like a lot, but this will also buffer the Sodium Bromine that I will add later. I will also add five 3 inch chlorine tablets to help replenish the chlorine during the rest of 79 the week. Next, I will add 3 lbs. of Potassium Peroxymonopersulfate. This will oxidize most of the organic material in the water and act as a water clarifier. This will also free up the chlorine to sanitize the impurities in the water. Finally, I would add 15 ounces of Sodium Bromine, usually a brand called “YellowTrine”. This will act as my algaecide. Let’s not forget the Conditioner. I would add 4 lbs. of Conditioner, through the skimmer while the pump is running, and allow the pump to run for an additional 4 hours. I would finish off by servicing the pool normally and brush the walls well to break up the algae and allow the chemicals to work. Expect there to be no chlorine in the water next week, since it will be used up recharging the Sodium Bromine. Example #2 Conditions: It is winter, the weather is cold but it has been sunny out. This is an average sized 20,000 gallon fiberglass surfaced pool. There was 3.0 Free and Available Chlorine reading. The pH Balance is at 7.3 and the Total Alkalinity is at 90 ppm (parts per million). The water is clear and there seems to be no problems. Actions: This pool seems ok, but being it is a fiberglass surface I know that on sunny days it will experience a more rapid degradation of chlorine than a regular pool. I would add 1 gallon of Liquid Chlorine. This will help keep the pH Balance up a bit and add enough chlorine to the water to survive direct sunlight conditions. I would not add any 3 inch tablets because in cold water the tablets will take over 2 weeks to dissolve, which is too long for a pool that is not being used. Also there is acid in 3 inch tablets and I try to avoid adding acid to a fiberglass surfaced pool unless I absolutely have to. Otherwise I would service the pool normally. Example #3 Conditions: It is late spring during the Spring bloom when there is plenty of pollen in the air. The pool is a large 30,000 gallon Pebble Tech surfaced pool with a salt chlorination system. The Free and Available Chlorine is a 5.0. The salt level is at 3300 ppm (parts per million). The pH Balance is 7.8 and the Total Alkalinity is 90 ppm (parts per million). Actions: The salt chlorination system is notorious for raising the pH Balance. Adding more Muriatic Acid on a regular basis to counter this continuously rising pH Balance has taken its toll on the Total Alkalinity. The Total Alkalinity is still at a safe level being at 90, however as more and more Muriatic Acid is added to keep the pH Balanced that acid will steadily lower the Total Alkalinity. I would add 1 gallon of Muriatic Acid to balance this pool’s water chemistry. If the Total Alkalinity were higher, perhaps 110 to 120, I would add more acid, perhaps a half gallon more, but since the Total Alkalinity is only 90, I know that the 1 gallon of acid I am adding will be sufficient to adequately lower the pH Balance and keep the chlorine in its killing form. I would service the pool normally otherwise. I might throw in a few lbs. of Sodium Bicarbonate as well to buffer the Total Alkalinity a bit if I have some extra on my truck. 80 Example #4 Conditions: It is late autumn and the winds have started to blow. The weather is still very warm. The pool is an old white plaster pool and spa, about 22,000 gallons and the plaster is pitted and stained. There is patchy Mustard (yellow) Algae along the wall in the deep end and in a corner in the deep end along the wall are also a few black spots, slimy nodules upon closer inspection and I realize it is the dreaded Black (blue-green) Algae. Oh no! What to do?! After testing the water I got a Free and Available Chlorine reading of 4.0. The pH Balance was at 7.5 and the Total Alkalinity is at 100 ppm (parts per million). That isn’t too bad for water chemistry, not bad at all really. Don’t feel bad. Algae can become chlorine resistant and even perfect water chemistry sometimes can not dissuade algae growth. So, what are we going to do about this? Actions: Even though this is only a slightly larger than average pool, I would start by adding 1 gallon of Muriatic Acid. This will lower the pH Balance very low, but not below 7.0, as there is enough Total Alkalinity to neutralize the acid. Even though the chlorine level was fine I would add another 3 lbs. of Dichlor granular (or 3 gallons of liquid) chlorine. This will increase the sanitizer and oxidizer as well as keep the Sodium Bromine charged when we add it. I’d add five 3 inch chlorine tablets to the floater as well, to make sure that chlorine is constantly being distributed into the water. I would then add 14 ounces of Sodium Bromine as an algaecide, changing the sanitizer and oxidizer to something the Mustard and Black algae have not yet experienced or built up a resistance to. I would also add 3 lbs. of Potassium Peroxymonopersulfate to aid as an oxidizer and to free up the chlorine as a sanitizer. I would then backwash the filter and after adding fresh D.E. powder I would add 1.5 liters of “Phos-Free” as a phosphate remover. When it comes to Black Algae, even if only in its infant stages, hold nothing back and attack it aggressively. I would also check to see if I have a Nature2 or PoolRX in place. If not, I would certainly consider adding one. In this case, due to the black algae I might even use some SilverTrine as silver is the most powerful metal based sanitizer we have at our disposal and is effective in killing off black algae. I would then scrub the walls, and especially the Black Algae vigorously with a steel wire wall brush. I do this to score and scratch the surface of these very durable algae, making it more susceptible to the chemicals. I would service the pool normally. Double check to make sure the pump is running for 8 hours a day. The Black Algae may take a couple weeks to get rid of as it is incredibly tough. Next week I would replenish the chlorine in the water, being that it will likely be gone from recharging the Sodium Bromine. Keep scrubbing away with the steel wire wall brush, keep the pH Balance a little lower than normal at 7.3 to 7.4 to keep your chlorine tenacious and aggressive, and keep the chlorine strong. Even Black algae will succumb to your will. Example #5 Conditions: It is winter time, cold and cloudy. The pool is a smaller sized 15,000 gallon 3M Color Quartz pool. The chemicals tested at a Free and Available Chlorine reading of 3.0. The pH Balance is at 7.4 and the Total Alkalinity is at 100 ppm (parts per million). I also checked the Conditioner level and found it to be at 90 ppm (parts per million). 81 Actions: Clean the pool normally. The chemicals are fine. Make sure the filter pressure is not too high and check the baskets for debris and such. The chlorine is fine at 3.0 as no one is swimming, there is not very much direct sunlight and the Conditioner level is fine. That chlorine isn’t going anywhere, the pH Balance is low enough that the chlorine is well in its killing form and that chlorine will last quite awhile in that cold water. Example #6 Conditions: It is late spring, the weather is warm and sunny and people are anxious to go swimming. This is a fiberglass surfaced, average sized 20,000 gallon pool and spa. The Free and Available Chlorine reading is at 2.0, the pH Balance is at 7.8 and the Total Alkalinity is at 60 ppm (parts per million). Actions: These are some unusual chemical conditions, but it can happen. First off, I would want to know that my chlorine is protected, being that I am getting such a low chlorine reading. I test for the Conditioner level and find it to be at 100 ppm (parts per million). The Conditioner is perfect so I know the chlorine is protected. Now I have the dilemma of a high pH Balance and a low Total Alkalinity. I would add 3 lbs. of Dichlor granular (or 3 gallons of liquid) chlorine. This may seem like a lot but this is a fiberglass pool during sunny conditions when people could start swimming. I would also add three 3 inch chlorine tablets to the floater. 3 inch tablets have acid in them, however the pH Balance is high enough to handle the added acid. I would add 1 quart of Muriatic Acid to lower the pH Balance, even though the Total Alkalinity is low. I would follow up by adding about 5 lbs. of Sodium Bicarbonate (Baking Soda) to increase the Total Alkalinity and therefore control the speed in which pH Balance changes. I would then service the pool as normally. 82 Playing Chess with Water Chemistry: The difference between a good water chemistry service technician and a great one is his or her ability to be a few steps ahead of water chemistry instead of behind it and constantly trying to correct it. Keeping your water chemistry perfect is like playing a game of Chess. You always want to be a step ahead of the game to win. A good technician can correct the water chemistry at the swimming pool at that time. A great technician can control what the water chemistry will be the next week by anticipating what the water will be and what it needs. When you test the water, don’t be as concerned with what the chemistry readings are today as much as to consider what you want the readings to be next week when you return. When you test the Free Available Chlorine and get a 3.0 reading, that is pretty good. While most swimming pool technicians will figure there is plenty of chlorine in the water and walk away, a great technician will consider what the chlorine reading will be next week. At this point, by adding just an extra pound (or gallon) of chlorine or an extra 3” tab or two this will ensure, depending upon swimmer usage, that next week the Free Available Chlorine reading will remain at 3 to 5 ppm and will still be good the following week. You don’t want any weakness in your chlorination system. If your chlorine drops to 1.5 ppm during the week and your pH balance raises as it always does than your chlorine won’t be able to sanitize, oxidize or keep algae from growing. Always plan ahead and consider what you want your chemistry to be, not just today, but the following week. When balancing the pH balance always remember that water’s natural pH is 8.0 to 8.2 and it will always try to return to that point. Also when a salt system is in use the chlorine produced by these systems has a very high pH of 11 to 13 and will be increasing the water’s pH all week long. Be sure to add Muriatic Acid to compensate for this expected and constant rise in pH. If your pH balance is 7.5 today that may seem fine, however don’t forget to consider what it will be the following week if you do not add acid now. If left on its own, that nice pH balance of 7.5 will raise to 7.7 by the following week, or more if a salt system is in use, which means that the pH is too high and will only allow 50% or less of your chlorine to be in its killing form. This high pH and weakened state of chlorine is a perfect opportunity for algae to grow, even though the water chemistry seemed to be ok. Even at 7.5 pH balance be sure and add that ¼ to ½ gallon or so of acid to keep the pH low. 83 Remember, a great technician is always ahead of the water chemistry, not behind it. Things to Remember About Section 18 Chemistry is one of the most important aspects of swimming pool service. Knowing the chemicals you have available and their proper application will set you apart as a professional technician and not just some pool guy. When in doubt about chemistry, your service manager or other resources are usually just a phone call away. Bad or negligent, even lazy chemistry can not only result in the loss of a customer but can lead to costly damage to the swimming pool’s surface, equipment and even harm to the swimmer’s healthy. Always remember that each service account is different, just like people. The human body is made up of about 75% water and each person has a little different chemistry and personality. You will find that swimming pools and water chemistry are not so different. 19. Desired Chemical Levels Reference Chart This reference section is designed as a quick reference chart to help maintain the desired balanced water chemistry in the swimming pool. This chart will go over Free and Available Chlorine (FAC), pH Balance, Total Alkalinity, Conditioner, Calcium Hardness and Salinity (Salt) as well as the chemicals required to increase or decrease these levels. Free and Available Chlorine (FAC): Free and Available Chlorine (FAC) should be maintained at 3.0 to 5.0 ppm (parts per million). Why? Chlorine is a sanitizer (kills germs and bacteria) and an oxidizer (burns away organic material such as swimmer waste). Warm temperatures, water turbulence and the Sun’s U.V. rays burn away chlorine, while germs, bacteria and organic material consume chlorine. Liquid Chlorine, 1 gallon will raise the chlorine level of an average sized 20,000 gallon pool by roughly 2.0 to 2.5 ppm of chlorine. Liquid Chlorine has a high pH and 1 quart of Muriatic Acid will be required for each 1 gallon of Liquid Chlorine to balance out the high pH effects. Liquid Chlorine is poured directly into the water and is safe to use on any surface. Dichlor granular chlorine contains both chlorine and conditioner. Dichlor is very close to being pH neutral and requires no Muriatic Acid to balance its effects. Dichlor granular is a quick dissolve product and is safe to use on any surface. 1 lbs. of Dichlor is roughly equivalent to 1 gallon of Liquid Chlorine. Trichlor granular chlorine contains chlorine, conditioner and acid. Trichlor granular is a slower dissolving product that should never be used on a dark plaster or other dark surface. Trichlor granular is usually sold as an algaecide, allowing the product to sit over top algae and burn it away. Trichlor granular is made from the same chemicals as 3 inch Trichlor tablets. 1 lbs. of Trichlor granular is roughly the equivalent of 1 gallon of Liquid Chlorine. 84 Trichlor 3 inch chlorine tablets are a slow dissolve form of Trichlor, made for chlorination systems such as a 3 inch tablet floater or a 3 inch tablet chlorinator. 3 inch tablets should always be kept inside the chlorinator and never placed in the skimmer. Three to four 3 inch tablets are about the equivalent of 1 gallon of Liquid Chlorine. 3 inch tablets will dissolve more slowly, over 2 to 3 weeks in cold water and in poor circulation. Calcium Hypochlorite granular chlorine is a more concentrated form of chlorine, however has a substantial calcium byproduct which will, with regular use, increase the Calcium Hardness of the water shortening its lifespan in the swimming pool. Calcium Hypochlorite is also nicknamed “dirty chlorine” because of its tendency to cloud the water and for its high concentration of calcium. Calcium Hypochlorite is added directly to the water and is safe to use on any surface. 1 lbs. of Calcium Hypochlorite is roughly equivalent to 1 ¼ gallons of Liquid Chlorine. pH Balance: The pH Balance of the water should always be maintained between 7.4 and 7.6. Why? There are two reasons for this. One is because the lower the pH balance is the more chlorine is in its killing form to sanitize, killing germs and bacteria and oxidize organic material. Water’s natural pH is 8.0, however chlorine is only at 0% to 5% of its killing form at that pH. At 7.6 to 7.7 chlorine is at 40% to 60% of its killing ability. At 7.4 to 7.6 pH chlorine is at 70% to 80% of its killing form. The second reason is that a human’s natural pH balance is 7.34 to 7.45. The closer the water’s pH balance is to that of human beings the more comfortable the water will be to swim in. To lower the pH Balance, add Muriatic Acid directly to the water. To raise the pH Balance, add Soda Ash (Sodium Carbonate) directly to the water. Total Alkalinity (T.A.): The Total Alkalinity of the swimming pool water should be maintained at 80 to 120 ppm (parts per million). Why? Total alkalinity is a measurement of alkaline particles in the water. These particles are what absorb the acid when you add muriatic acid to the water to lower the pH balance. These alkaline particles are the only thing between the acid and the pools surface and equipment. They neutralize the acid so that the acid can be added safely to the water, without risking damage to the pool’s surface, equipment or the swimmer. When the total alkalinity is too high, the acid is absorbed and neutralized too quickly and the pH is not lowered effectively enough. If the total alkalinity is too low than the acid is not absorbed and neutralized quickly enough causing the pH balance to drastically lower and the water to become corrosive and damaging. To lower the Total Alkalinity, add Muriatic Acid directly to the water. To raise the Total Alkalinity, add Sodium Bicarbonate (Baking Soda) directly to the water. Conditioner, Stabilizer (Cyanuric Acid): 85 The Conditioner level should be kept at 70 to 90 ppm (parts per million). Why? Conditioner protects your chlorine from the Sun’s harmful U.V. rays. If your Conditioner level is low than your chlorine will not be very well protected and you will get “chlorine bounce” which is when your chlorine is strong the day you add it but is destroyed quickly and is weak or gone by the time you return the following week. When the chlorine bounces in this manner algae has the opportunity to grow while the chlorine is weakened and therefore becomes VERY resistant to the chlorine, since it had bloomed with chlorine in the water. If chlorine resistant algae blooms in the water than you must change the sanitizer to Sodium Bromine to kill it off. Conditioner level will be lowered by 20 to 30 ppm by the end of the summer just from heavy usage and splash so be sure and check it before, during and after the heavy swim season to make sure your chlorine is always as protected as it can be. Too much Conditioner can cause staining while not enough Conditioner will cause chlorine bounce and unstable chlorine. To increase the Conditioner level, add Conditioner slowly through the skimmer while the pool pump is operating and allow it to circulate, breaking down inside the filter for 4 hours. To decrease the Conditioner level, the pool water will have to be drained. Calcium Hardness: The Calcium Hardness should be kept from 180 to 500 ppm (parts per million). Why? When the Calcium Hardness level is too low than the water will seek out a source of Calcium, becoming corrosive and will pull it right out of the plaster, grout and concrete on the deck when it splashes. When Calcium Hardness is too high it can cause scale on the surface and at the water line, clogged filters, unbalanced chemistry, unhealthy water conditions and even damage to the heater. To increase the Calcium Hardness of the water, add Calcium Elevator. To decrease the Calcium Hardness of the water, the pool water will have to be drained. Salinity (Salt): For salt chlorination systems only, the Salinity (Salt) level in the pool water should be kept from 3,000 to 3,500 ppm (parts per million), however always check with the manufactures recommendations. To increase the Salinity (Salt) in the pool water, add salt directly to the water and brush it evenly around the surface with your wall brush for dissolution. 50 lbs. of salt will raise an average sized 20,000 gallon pool’s Salinity 300 ppm (parts per million). To decrease the Salinity (Salt) in the pool water, the pool water will have to be drained. Phosphates: 86 Phosphates are microscopic dead plant material and provide a ready food source for algae. The average pool will see an increase in its Phosphate level of about 50 ppm per week, however this depends greatly on the amount of debris around the pool and how much is being introduced into the water. Preferably the Phosphate level should be at 0 ppm at all times, however this is quite unrealistic. Try to keep the Phosphate level below 200 ppm if at all possible. To decrease the Phosphate level in a pool you will have to use a Phosphate removing agent such as “Phos-Free” or “Starvers”. There are actually many different Phosphate removers in the market to choose from. When doing an initial Phosphate removal treatment you will want to backwash the filter (if a D.E. filter) first before adding the Phosphate treatment. Then, after 24 to 48 hours you will have to clean the filter completely. Phosphate removers cause all the Phosphates in the water to clump up into a slimy film and with circulation and vacuuming get trapped in the filter. These clumped up, slimy Phosphates can drastically increase the filter pressure so always be sure to clean the filter afterwards. For a regular weekly Phosphate removal treatment you will add small amounts (see directions on the product) to the water each week. This will help keep the Phosphate level down and will cause algae to starve. The “Four Pillars” Algaestat/Algaecide Program: For an excellent regular maintenance program see “Section 11, page 50” for the “Four Pillars” Algaestat/Algaecide regular maintenance program. Remember to always stay ahead of your water chemistry, not behind it. 87 20. What is Expected of a Service Technician So, at last we have come down to it. After speaking about equipment, chemicals, algae and such it is finally time to talk about what all this means to you and more so, what is expected of you as a service technician. Professional Behavior: As a self employed service technician of you are expected to exhibit courteous and professional behavior to your customers. This means: Service Note A customer will often keep a service technician around despite poor service simply because they like the technician. By being personable, professional and hard working you are giving your customer many reasons to keep you on for service. Always be attentive to your customers needs. Always be presentable during work hours. Be mindful of your tone and language when in the presence of the customer. Even if it is a long time customer that is acceptable to the use of bad language always try to remain professional. This also includes when you are on your cell phone. Always try to remain calm and professional when communicating with a customer, even if they are upset and complaining. Remember that you are the greatest asset to your company. Represent yourself and your company with dignity, courtesy and professionalism. Communication: 88 It is said that communication is the foundation to any relationship, whether it be a friendship, marriage or business. As a service technician you are expected to communicate in many different ways. Always do your best to keep your scheduled appointments, whether it is with customers or with other sub-contractors. As a professional courtesy, call if you are going to be late. Sometimes things happen and not all appointments can be kept. Extend the professional courtesy to communicate so as to not inconvenience others time. When you are in the field it is a good idea to leave a door hanger with your name and the date on the customer’s door to show that you have been there. Be sure to write down any pertinent notes on the door hanger, such as to add water or if a leak was found. A door hanger is usually the first thing a customer will see when they return home and having that in place can prevent many calls of complaint. Always sign in to the service log at the time clock if the account has one and if you as a company use one. The service log is a handy communication tool and should have such details to sign in as the technicians name, date and chemical readings. Service Note As a service technician, you are the eyes, ears and voice of your company in the field. You are there with the customers and their pools every day. Your ability to communicate anything from repairs needed, chemical readings, customer’s requests and more are vital to your company. Always sign in to the service log in your vehicle. Each service vehicle should have a service log to register the customer, date, chemical readings and what chemicals were added that day. This document is important to you for the sake of liability. Should you, as a company, need to know the chemical conditions on a particular service stop or the chemical conditions and changes over a period of time than this service log is your only form of accountability. Always communicate with your customers. If there is a leak in the equipment or plumbing, if the water level is low on a regular basis, if there seems to be some new discoloration or staining on a pools surface or if a pool could use an acid wash, if a gate is broken or for any other unusual service or safety related issues always be sure to contact your customer to address the issue. While service helps a company to survive it is repairs and extra maintenance that help it thrive. Always communicate with the customer regarding any regular maintenance items, such as pump baskets, skimmer baskets, weir gates, floor cleaner hoses or repairs that need to be repaired or replaced. Always be sure to offer your extra services to the customer. You are also the sales representative in the field. If a filter needs to be cleaned than offer a Phosphate treatment before hand since the filter is required to be cleaned anyhow. Offer to provide the customer with Phosphate remover for regular weekly maintenance. If their pole is old or their net has holes in it offer to replace them. During the swim season you may want to remind them that 89 you might also sell pool toys. Always try to educate the customer on the hazards of old water to keep them draining the pool every 3 to 5 years. If they do not understand the hazards of poor quality water than they will be reluctant to want to drain and refill. Service Technician Duties: As a service technician you are responsible for many duties. The duties we will be going over here relate to service in the field as they pertain to the average full service account. There are some specialty service accounts such as spa only service, chemical only service and chemical plus or chemical premium service. Always keep the gate closed, whether there is a dog or not, in the back yard and the pool area. Get into the habit of always closing the gate. If the gate is not working properly immediately report it to your customer. Keeping the gate closed at all times will reduce the chances of a child gaining access to the swimming pool area unattended or a pet escaping the yard. Caution! ALWAYS close the gates behind you! A negligent service technician is an inexcusable offense when faced with the potential of a child drowning. Also, when a pet is lost it is amazing how quickly a $50 mutt from the pound becomes a $5,000 member of the family. Always check both the skimmer and pump baskets to keep them clear from debris. If those baskets break it can send large amounts of debris through the plumbing and can even damage the pump. Put the debris in a trash can. Do not dump it there by the equipment. Make sure any 3” Trichlor tablet floaters are tied down. Be in the habit of having string with you for just this reason. An unsecured floater poses a risk to swimmers who jump into the pool as well as when the floater sits over the top step of the pool, the acid that dissolves from the tablets can cause damage to the surface. It is usually a good idea to test for and add chemicals to the pool at the beginning of your service. This way the chemicals are well distributed in the water and the swimmers can return to the pool once you complete service. Don’t forget to brush the tile with a tile brush and tile soap. Brushing the tile will clean the scum line and free any debris along the tile to make it easier to net out. Brushing the tile will not remove calcium but it will help keep the tile clean from other debris. You should always net the top and bottom of the pool of debris, leaves, sticks and such. It is usually a good idea to remove any pool toys from the water before doing this. Put the toys someplace out of the way as to not trip over them while servicing the pool. 90 Vacuuming the pool is often considered the most tedious and undesirable of the service technician’s duties. A customer usually associates vacuuming with getting full service and even if the floor of the pool is spotless and dust free a customer will still call the office and complain that “the pool wasn’t vacuumed. I want better service!”. Vacuum the pool as needed and especially when the customer is home watching. We understand that it is simply not realistic to vacuum the pool with every service, however leave no reason for the customer to call regarding a dirty bottom pool. A Note About Vacuuming Vacuuming the pool at every service? Sure, it is easy for some guy writing a book or a home owner to expect a service technician to vacuum the pool every week. All too often do customers complain about the service technician only being in the backyard for 5 minutes and not vacuuming. Customers usually relate vacuuming with getting full service. Realistically however, vacuuming every week simply isn’t going to happen. Do your best to keep the pool free from dust and the surface looking good. If the customer is home and watching than give it a good vacuuming, if nothing more than for performance sake. Do not, however give the customer a reason to complain due to not vacuuming. Brushing the walls should be done every time to discourage algae growth and to brush free any dirt that may be clinging to the walls to vacuum up the following week. Brushing the walls is fairly quick and vigorous making it easy for the customer to notice. It is a good idea to keep all of your equipment together when servicing a swimming pool as to not forget or lose any equipment when you leave. Always inspect the pool and equipment. Be sure to report any leaks, any plaster that could use an acid wash, any mastic in need of repair or other repair issues to the customer. Remember that while service is what helps a company to survive, it is repairs that help a company to thrive and grow. If you finish your service quickly, find extra things to do such as straightening up the outdoor furniture around the pool area or picking up any towels left lying around. Anything that makes the pool area more presentable will enhance the service experience for your customers and set you apart as a professional service company. If the filter pressure is high and you backwash the filter always be sure and clean up any remaining diatomaceous Earth left around the skimmer. Never leave a mess of any kind in the customer’s back yard. Helpful Documents to have Prepared: You will need to keep various documents with you as a service technician. Some of these documents are fairly common while others will require additional classes and testing. Driver’s License. You are required to have a current and valid Driver’s License to drive in the state of California. 91 Proof of Vehicle Insurance. You are required to have Proof of Vehicle Insurance to drive in the state of California. Material Safety Data Sheets (M.S.D.S.). You are required to have the Material Safety Data Sheets for each chemical you carry on your vehicle due to the dangers of a hazardous materials spill or accident. This is for your safety and for the safety of those around you. As a service technician you carry in your vehicle some very dangerous chemicals. It is best to be responsible in the handling and transport. If you do not have the Material Safety Data Sheets on your vehicle than you can find many of them at your wholesale supplier or by visiting www.hasapool.com for a copy of the ones you need. I recommend keeping them together in a properly labeled folder. Certified Pool Operator (C.P.O.) certification. Being C.P.O. certified shows that a service technician is serious about his or her profession and is properly certified to be operating a customer’s swimming pool. If you do not currently have a C.P.O. certification than you might consider becoming certified by visiting the National Swimming Pool Foundation website at www.nspf.com. There are usually classes to be taken as well as some testing. This is an excellent certification to have that most service technicians in the swimming pool industry do not actually possess. Being certified sets you apart from the competition and distinguishes your company as being truly professional. Water Chemistry Certification. Being water chemistry certified requires far less time and testing than becoming C.P.O. certified. Its main focus is on chemistry. To become water chemistry certified you must first read and study from the “IPSSA’s Basic Training Manual: Part 1” which is offered by the Independent Pool and Spa Association. The book can usually be purchased at your local wholesale supplier or you can find it here http://www.ipssa.com/index.php?option=com_content&task=blogsection&id=7&Itemid=37. The test itself is online. In actuality, most service technicians in the industry are not water chemistry certified. If you are not currently certified than it something you should give some serious consideration to achieving. Becoming water chemistry certified will set you apart from the average service technician and as a company it shows that you are proficient and certified in chemistry which will offer our customers peace of mind and confidence in your abilities. 92 Things to Remember About Section 20 The service technician is the backbone of any swimming pool service company. They are the representatives and the ambassadors of good, quality and competent service. They are the eyes, ears and voice of the company that keep operations running smoothly. A service technician has many duties and expectations. With some training, practice and experience you will be able to execute your duties quickly, efficiently and skillfully. 21. Specialty Services (Chemical Only and Spa Service) On occasion you will have an account that is only chemical service or just a spa. While the principles of service remain the same there are some differences in these specialty forms of service. This section covers those differences. Chemical Only Service: Some customers only want us as a company to take care of the chemicals while they handle the rest. You will more often than not find these accounts to be far less maintained than if they were full service as the customer tends to be unskilled and often lazy when it comes to the care of their own swimming pool. You will also find algae growth more frequent at these types of service accounts as the customer will often try to cut expenses by doing things such as turning the time clock down or completely off offering the pool little or no circulation or filtration. When algae begins to develop they are usually quick to blame the chemicals. Here are a few extra things you can do to keep the pool safe and the customer happy. The chemistry at a chemical only service remains the same as a full service account. Always remember to leave a door hanger with your name and date on it as well as sign into the service log by the time clock if the account has one. If the furniture around the swimming pool is in disarray and you have extra time, as a courtesy it is always good to straighten it up a bit. This will make it more noticeable that you have been there and that you are willing to take the extra effort to provide quality service. 93 Always inspect the customer’s pool equipment and leave them notes often. Should you notice that the skimmer or pump basket is full than go ahead and empty them to prevent any harm or damage to the equipment, however be sure to write the customer a note on the door hanger stating that you provided such service and that they should be mindful of maintaining their equipment to prevent costly repairs. Should you notice that their pool is neglected in service than be sure to remind them that you do offer full service as well. Sometimes an account will be considered “chemical service plus” or “chemical service premium” which usually means that you will also clean out the skimmer and pump baskets and may net out debris or brush the walls. Spa Service: There are as many different kinds of spas and spa surfaces as their all pools and pool surfaces. Spas can be much easier to service or sometimes more difficult. There are many things to remember when servicing a spa. This section will help you to navigate around any hazards when conducting spa service. First, here are a few notes regarding plaster in-ground spas. Plaster Spas: Plaster, Pebble Tech and 3M product surfaces are quite forgiving when it comes to chemical balance. Just as a plaster or similar type of surfaced pool, in a spa the surface will give off Alkaline material to help keep the Total Alkalinity fairly balanced or at least, easier to balance. Most of these inground spas are not covered and should be chemically sanitized and oxidized with a few 1” Trichlor tablets or a single 3” Trichlor tablet about once every other week or as needed after determined by proper chemical testing. Caution! Never use more than a handful of dry chemicals or a cup of liquid chemicals at a time in a spa. Too much chemicals can have a drastic change on the chemistry, can cause damage to the surface and equipment and can be unhealthy for swimmers. Did you know? Two adults can use up all of the sanitizer and oxidizer in a spa in 15 minutes. It is always good to use a floater or some form of chlorinator in a spa to help maintain the sanitizer and oxidizer level. Muriatic Acid should still be added in VERY small amounts, no more than a cup at a time to reduce the pH balance as needed. Conditioner should also be added in VERY small amounts. It only takes just a little under ½ lbs. of Conditioner to chemically start up the Conditioner in a newly filled spa. Be very mindful of the pH Balance and Total Alkalinity in a spa. It can change quickly. 94 Copper and Copper Oxide Staining in In-Ground Plaster Spas: If an in-ground plaster, pebble tech or 3M product spa has old copper plumbing and/or an old heater there is a SUBSTANTIAL risk of copper and copper oxide staining. All spas should be drained and refilled regularly once every 6 months. In the case of older spas with copper plumbing and old heaters the friction Caution! of the water as well as the wide range of chemical changes in Do not use Trichlor granular such a small body of water can strip copper from the plumbing (Algae Ban), or Calcium and, after enough accumulation deposit it onto the surface of Hypochlorite in a spa where the spa. copper staining is present. It will oxidize the copper stains turning Copper will manifest itself with light to dark turquoise and blue them grey to black. stains. When copper is oxidized by chlorine it will turn gray to black. Sometimes this chemical change will happen as you add chlorine right before your eyes. It can be very sudden and drastic. If you have an older spa with copper plumbing be VERY careful to not over chemically treat it, else the staining results can be most unfavorable and severe. Fiberglass and Acrylic Spas: Fiberglass and Acrylic spas, whether above ground or below can be VERY sensitive to chemical changes. Like any spa, two adults can consume all the chlorine in a spa within 15 minutes so keeping them chemically balanced with a once per week visit is near impossible if it gets used with any regularity. If the spa is covered than it is best to use Sodium Bromide 1” tablets as your primary sanitizer and oxidizer as Sodium Bromide, unlike chlorine will sanitize and oxidize regardless of the pH Balance. If the spa is covered than the Sodium Bromide will be protected from the Sun’s U.V. rays. If it becomes necessary to add acid to a fiberglass or acrylic do so only in the smallest amounts of ¼ cup at a time. The slightest amount too much of Muriatic Acid can turn a fiberglass or acrylic spa’s water acidic easily as they are VERY sensitive to pH change. This should never be necessary if you are using Sodium Bromine as your sanitizer and oxidizer. Be sure to keep 1” Sodium Bromide tablets handy as well as Soda Ash and Sodium Bicarbonate when servicing fiberglass or acrylic spas. Here are some good guidelines to follow when servicing an above or below ground fiberglass or acrylic covered spa: On average you will use 3 to 4 1 inch Sodium Bromine tablets per week, usually placed in a floater or in a dispenser located on the skimmer weir gate. You may use a couple more as needed in cases of heavy swimmer use. Spa Cover Note It is a good idea to use a garden hose to clean off the spa cover about once or twice per month, depending on how dirty it gets. On average, to assist on oxidizing organic material you will use 3 to 4 1 inch quick dissolve Potassium Monopersulfate tablets. These can be put directly into the spa and will dissolve within a few minutes. It is usually good to turn on the jets after 95 doing this to assist with dissolving and dispersal. The tablets will not harm the surface or cause staining. When you notice foam starting to build up it is good to add 3 to 4 ounces of liquid spa “Foam down” in order to remove the organic material build up that is causing the water to foam. About once a month or as needed you want to add a couple squirts across the surface of a spa algaecide. Leisure Time makes an excellent non-foaming liquid spa algaecide that uses the active ingredient: Poly [oxyethylene (dimethyliminio) ….. 6% About once a month or as needed you will also want to add a couple squirts across the surface of an enzyme based oil remover. This will help keep the oils and scum line build up down across the surface of the spa. Keep the water level just below the head rests, else the chemicals will deteriorate the head rests over time. The pH Balance and Total Alkalinity will fluctuate and change quickly in a fiberglass or acrylic spa. Always have enough Soda Ash and Sodium Bicarbonate to keep the pH Balance and Total Alkalinity maintained properly. Some fiberglass and acrylic spas are equipped with a Nature2 (spa size) water purifier and sanitizer. As we know this is a metal based sanitizer and the cartridges should be replaced every 4 to 6 months. If a Nature2 cartridge is not installed on a spa you can also use a spa sized PoolRX which works the same and can be placed in the skimmer. The PoolRX cartridge should also be replaced once every 4 to 6 months. When a Nature2 or PoolRX cartridge is in use on a fiberglass or acrylic spa it is good to keep in mind that these metal based sanitizers only sanitize. They do not oxidize the water. You will still need to use Sodium Bromine or Potassium Monopersulfate to oxidize the water. Equipment to use for fiberglass and acrylic spas: Being that spas are small and are often in smaller enclosed areas it can be useful to have equipment especially suited for spa service. A spa can be in a corner, within a gazebo or surrounded by other decorations, plants and such. Standard pool service equipment can be large and cumbersome in these cases. Here is a list of some useful equipment for the care of a fiberglass or acrylic spa account: Did you know? A shortened pole and spa net for skimming the surface of debris A hand held tile brush for cleaning the scum and oil off the water line of the spa A hand held spa vac or spa wand. These are useful vacuums that rely on motion created suction or on water 96 A spa, whether it be fiberglass, acrylic, plaster or otherwise, indoor or out door, above ground or below ground it should be drained and refilled every 6 months without fail. The chemistry changes far too often in a spa for the water to remain healthy for overly long. motion to create suction to clean up dust and debris off the surface. They do not require a vacuum hose or suction generated from the pump as they are quite self contained. “303” sealant and surface protector and a rag to apply it. 303 is a water sealant and surface protector that helps to protect a spa cover and the wood siding of a spa from moisture and also helps to keep it looking clean and vibrant. This should be applied using a rag and rubbed across the cover and wood siding as needed, usually about once per month. If you do not currently have this in your inventory than ask your local wholesale supplier what types of spa cover protection products they have in stock. A bucket or a small hand held equipment cart is useful for carrying your equipment and chemicals to the spa. A submersible pump, 50 to 100 feet of hose and an extension cord to drain a spa as needed. Draining and refilling a fiberglass or acrylic spa: A spa, no matter what kind should be drained, refilled and the chemicals replenished every 6 months. A service technician will usually be responsible for the draining, refilling and chemical start-up of a fiberglass or acrylic spa. These are some guidelines to help you to properly drain, refill and chemically start-up a fiberglass or acrylic spa: Caution! Always use caution when draining a pool or spa. There are many things that can go wrong in this process such as the drain water overflowing, water getting to an electrical connection, forgetting to turn the power off to the time clock and the pump turning on when the pool or spa is empty and other such hazards. Always take every step available to drain a pool or spa as safely as possible. When draining the spa always make sure that the extension cord is plugged into a plug with a G.F.I. to prevent any accidents. Try to keep the connection part of the extension cord off the ground to protect it from moisture or any near by sprinklers. Always keep safety in mind when using electrical devices in water. When you drain the spa be sure to drain the water into a dedicated sewer line whenever possible. If there is no sewer line available to drain the water into that attempt to drain the water into a deck drain to be sent out to the street. A spa should drain fairly quickly, depending on the size of the submersible pump. Always try to keep the hose and extension cords out of the way of walking traffic to prevent accidents. Once empty, should the customer request it as it is an extra charge, there are acrylic polishes that can be used to polish and protect the acrylic surface. Ask your customer if they want this done before charging them extra. If there is no submersible pump available to drain the spa you can use a garden hose to siphon out the water as well, although this will take longer. After the spa is refilled the chemical start-up should consist of: 3 to 4 1 inch Sodium Bromine tablets placed in a floater or in the compartment on the weir gate, 3 to 4 ounces of granular quick dissolve Sodium Bromine added directly to the water, 3 to 4 ounces of quick 97 dissolve or 3 to 4 1 inch quick dissolve Potassium Monopersulphate tablets added directly to the water, 2 squirts across the surface of a liquid non-foaming spa algaecide, 1 16 ounce bottle of a stain Sequestering agent that will prevent the metals in the fill water from falling out of circulation and cause staining. Things to Remember About Section 21 Even though chemical service and spa service can be completely different than full service always remember to keep the customer in mind and offer the highest quality service you can to represent yourself and your company well. Safety and common sense play an important role when servicing a smaller body of water such as a spa. Always keep close watch on the ever changing chemical conditions. 22. How to Calculate a Pool’s Volume (Gallons) Knowing your swimming pool volume is important information to be able to balance your pool water chemistry, sizing pumps and filters. Steps: 1. Measure the swimming pool. You will need the overall length, width and the depth in both the shallow end and deep end. The formulas below assume you are measuring in feet, and want results in gallons. 2. Use the appropriate formula below for your pool's shape. Rectangle Pool: (Deep End + Shallow End) / 2 = Average Depth Average Depth x Length x Width x 7.48 = Volume in Gallons Round Pool: Depth x Diameter-squared x 5.9 = Volume in Gallons Free Form Pool: (Deep End + Shallow End) / 2 = Average Depth (Width A + Width B + Width C + ...) / (number of measurements) = Average Width Average Depth x Length x Average Width x 7.48 = Volume in Gallons Warnings: If your pool is of extremely irregular shape, it would be wise to take as many evenly-spaced width measurements as you can stand, perhaps one width measurement for every foot of length. 98 Things you’ll need: Measuring tape Calculator Pen and paper Someone to help with the measurements 23. Safety Revisited Safety is a large and substantial topic to discuss. There are so many various aspects to safety that it can be a truly overwhelming topic. I will address each aspect of safety in chronological order from the start to the end of your service day. The start of your day: Before you leave for work make sure that you have all of the Material Safety Data Sheets required on your vehicle. These are kept in case of an accident so that a Hazardous Materials Response Team will have the information for each chemical that you have on your vehicle. With so many oxidizers, acid and other harmful chemicals an accident could be very dangerous to anyone around you. If you do not currently have these Material Safety Data Sheets than you can find them at your local wholesale supplier or online. Make sure the containers and equipment on your vehicle are tightly secured to avoid load shift or a loss of equipment when you are driving. Make sure your vehicle is properly inspected and in safe working order. Make sure you have plenty of water to drink, Did you know? The “7 Eleven” convenience store sells a plastic insulated drink container in Super Big Gulp and Double Gulp sizes. These drink holders are very inexpensive to refill, hold a lot of liquid and can your drink cold and ice solid for 10 to 12 hours. These things are excellent to have, especially during the hot summer months. 99 Caution! As a service technician, the Sun will bombard you from above as well as reflect the sunlight off the surface of the water while you are working. Always protect yourself by staying well hydrated, using plenty of sunscreen and by wearing a hat when servicing in the summertime. especially in the Summer. Protect yourself from dehydration and heat stroke. Use a large hat to protect yourself from the heat. In the Summer time the heat can become quite intense. Use plenty of sunscreen. Skin cancer after long time unprotected exposure to direct sunlight is a common problem in the swimming pool industry. Once you start driving: Make sure your head lights are on at all times to make your vehicle more visible to other drivers. Driving with your head lights on will reduce the chances for an accident. Always drive safely, obeying all traffic laws. If you must use your cellular phone use a hands free device or pull over to the side of the road and never text message while driving. When you arrive at your service account: Be careful to not allow moisture from rain or otherwise into your dry chemicals. Trichlor tablets and Trichlor granular have a mixture of both chlorine and acid. Moisture can deteriorate Trichlor freeing the chlorine and acid and mixing them in an enclosed container. The concentrated gas is known as “Mustard Gas” and can be highly dangerous. Also be careful when opening a 3 inch Trichlor tablet feeder as if it is clogged or not working properly than this “Mustard Gas” can also be trapped within the chlorinator. Moisture mixed with Calcium Hypochlorite can also be dangerous as it is such a powerful oxidizer, when mixed with water it can generate enough heat to catch fire. A Note About Parking If possible do not park in the customer’s drive way. This will prevent any possible oil leaks from staining their drive way and should the customer return home than they will not be inconvenienced by your vehicle being in the way. Sometimes there is no other parking available but if you can avoid it, stay out of their drive way. When using Liquid Chlorine and Muriatic Acid always be sure to dunk the bottom of the container in the water before and after use to prevent any unseen drips from staining the customer’s deck. If you must walk on the tile to service a pool always walk flat footed and slowly. The tile can become dangerously slippery when wet and a slip and fall on the tile can cause great injury. 100 Always walk carefully and slowly if you must navigate around artificial rock wall areas to service the pool. Artificial rock surfaces can be very uneven and difficult to maintain your balance on. Always be mindful of where your pole is. With a single wrong step or a moment of inattention can put your pole through a window or worse. Always! Always! Always! Close the gates behind you. Even if there is no pet in the yard be in the habit of always closing the gate to prevent children from straying into the pool area unattended and to prevent pets from escaping the back yard. Anytime a gate does not close or operate properly be sure to bring it to the home owners attention and contact your service manager regarding this safety hazard. Always be mindful and careful of where you lay your equipment on the deck. Customers do tend to come outside and your equipment spread out on the ground can cause the customer to trip. Try to keep the skimmer lid on the skimmer as much as possible. Often enough has a service technician accidentally stepped into a skimmer which can cause injury. In rain or wet conditions try to make sure that your hand is dry when turning on a pump, time clock or other electrical devise. An ungrounded wire or improperly installed electrical devise can cause an electrical hazard and injury to you. Should you come across such an electrical problem be sure to report it to your customer immediately. If a dog in the yard seems aggressive and unsafe do not try to enter the yard to provide service. Leave a note on the door hanger and report the problem and the inability to provide service to your customer. 101 24. Additional Notes About the Pool Service Business Becoming a swimming pool service technician and a small business owner means that you have many choices before you. When I first started in this business it took me a while to rewire my brain to understand that I owned the business. I kept asking the people whom I hired to help me build my service route “what do I do about this problem or that problem?” Their response was, “that’s a good question. What are you going to do?” It was then that I realized that owning the business meant that I held sole responsibility. I could change it and make adjustments as I see fit, but I also take responsibility for the choices I make. Operating your own swimming pool service business you will have to decide things like if you want to pursue repairs or subcontract them out. Do you want to send your bill to your customers in advance to fund your chemicals and expenses before the work is done, or after the work is completed when the pay has been earned? How will you reserve your vacation days? There are many technical problems you will have to solve with billing paperwork, invoices and such. As the owner of a business you will have to be the technician, the marketing and advertising department, the I.T. department, customer service, the secretary, the plumber, the electrician and more. The bottom line is that running a business isn’t easy. This section will discuss the operational aspect of the swimming pool service as a business. When should you send the bill to your customers? This is a commonly debated topic among swimming pool technicians. There is no right answer, honestly. Use the business model that works best for you. Billing your customer in advance at the beginning of the month before the work is done will generate income for you to pay for the chemicals and equipment you will need over that month, however some customers have fears that a pool guy might take the money and run, or once they have payment they will do lower quality work since they have already been paid. Billing after the work is done at the beginning of the next month makes you earn it by providing quality work before payment is made, however you must then rely on your customer to pay the bill since you are now out on chemical costs and labor. You 102 can also bill in the middle of the month and have the bill due at the end of the month. Each way has its pros and cons but ultimately it will be your choice. What insurance carrier should you choose? There are a number of “pool guy groups” that get together into a large organization for insurance and support. A couple to name for example would be the Independent Pool and Spa Service Association (I.P.S.S.A.) and the United Pool Association (U.P.A.). These “chapters” are divided up by geographical area. Each chapter will have their own dues to pay as well as the insurance dues to pay. The monthly prices for chapter dues and insurance will vary from one group to another, but figure on paying $ 75.00 to $ 90.00 a month (This estimation is made in 2010 and may not reflect future changes in prices). A group or organization will sometimes offer additional benefits such as getting you “Water Chemistry Certified” or offering “Sick Route Coverage” in the event you become ill or are unable to service your accounts. There is also a group called A.S.A.P.P. which does not require any meetings and is less expensive however they do not offer any sick route coverage. Each group or organization is different and may have a variety of benefits to offer. These groups are also a great resource for repair technicians, subcontractors, contractors and furthering your swimming pool business education. Usually you would be expected to attend an hour or two meeting once a month, but again each chapter and organization is different. Here are some online resources to find the groups nearest you: Independent Pool and Spa Service Association http://www.ipssa.com/ United Pool Association http://www.unitedpoolassociation.org/ American Spa And Pool Pros http://www.asapponline.com/ Should you advertise? I have never advertised in the yellow pages or other publication, however through larger companies I have experienced that the larger publications are excellent for bringing in repair business. I have been told by other technicians that the cheaper the publication you advertise in, so too will be the cheaper customers I have sent out door flier types of advertisements. Out of the 10,000 advertisements I had sent out I received about 8 customers. Fliers work better with repetition and timing. The more you send a flier to the same areas the more likely you are of getting a response. To gain new business I would recommend using large advertisements on the side of your truck. You can try the magnets however due to their lack of size and visibility these tend have gotten few responses. Word of mouth has been my most effective means of gaining new customers. I usually offer a month of free service for any references from my existing customers and also advertise this in my monthly billing statements. 103 There are many means to market and advertise your business though. Trial and error can be quite the educational experience though costly at times. Try to make the most you can with the least expense. Should you use a pool equipment cart? I tried using a pool cart for my first two years in business. For me it seemed a bulky and clumsy additional weight both in the back yard and on the back of my truck. I did not like the idea of having chemicals in the cart, outside of the bed of my truck. I also found that there were many yards that often had obstructions blocking my path with a pool cart to the swimming pool. While many swimming pool technicians swear by their cart, personally I prefer carrying my gear to the pool side. 25. Helpful Pool Service Forms and Documents I have placed here 8 different forms and documents that have helped me in the swimming pool service. On many of these forms I have placed the fictitious name “Acme Pool Service” which is shown here only in example and bears no reflection to any existing business. These 8 forms and documents I have personally used while in business. They were custom made using a very simple “Microsoft Word” program. There are many different accounting programs to help with your business paperwork, literature, billing, forms and other documents and these forms and documents that I am presenting here are only for use as an example. These forms and documents are as follows: Proposals for Swimming Pool and Spa Service: These are some helpful forms that I use to propose my business and prices to a new potential customer. There are 3 of these forms that I use: Residential Swimming Pool Service, Residential Spa Service and Commercial Pool Service. Wind and Rain Advisory: A form I use to alert customers of what I can and can not do during wind and rain conditions and ways they can keep their pool safe during these conditions. Summer Health and Safety: A form I send out in the summer to alert my customers of important health and safety tips. 1 Month of Free Service: A form I use to alert customers of my reference policy of giving 1 month of free service for each reference. Vacation List: A list of all the holidays that I will be taking off for the year. 104 Example Invoice: This is an example of a bill I would send to a customer at the end of the month. Water Chemistry Field Analysis Sheet: This is a handy form that I use for analyzing a new customer or a commercial account’s water chemistry. I would usually leave this with the customer to give an example of what their water chemistry should be and what it currently is. Swimming Pool Drain Waiver: This document is for use when you are draining the swimming pool or doing any repairs that require draining. This document should be signed and dated by the customer before the swimming pool is drained. *Please note that all of these forms and documents are here as examples only. Acme Water Pool Service Quality Pool and Spa Service since 1985 Residential Pool and Spa Service Agreement Service Includes: 1. 2. 3. 4. 5. 6. 7. 8. 9. Test water chemistry: chlorine, pH balance, total alkalinity, cyanuric acid, calcium hardness and phosphate levels. Adding chlorine and Muriatic acid. (Salt for salt generated chlorinators will be bill separately unless provided by the customer.) Clean tile, dirt and grease line only (calcium removal will be charged extra upon request). Net the top and bottom of leaves and debris. Vacuum the surface as needed. Brush the walls and surface as needed. Clean out the skimmer and pump baskets of debris. Inspect the equipment to insure that all is in working order. Backwash the filter as needed. Algaecide and Conditioner: 1. 2. Algaecide will be added all throughout the year but will be billed separately at $ 75.00 once in June. Conditioner will be added all throughout the year but will be billed separately at $ 40.00 once in February and once in September. Salt Chlorine Generators: 1. Salt chlorination cells for salt chlorine systems will be inspected every 90 days and cleaned as needed at $ 20.00 per cleaning. Filter Sanitations: 1. 2. 3. Filters will be sanitized, inspected, cleaned and parts lubricated as needed at a charge of $ 85.00 per filter for Diatomaceous and Cartridge filters. Filter sanitations are done once every 6 months. Sand filters can only be backwashed. To open a sand filter and stir the sand or replace the sand will be an extra repair charge. Pool Covers: 1. 2. Pool covers should be removed on service days to provide the technician access to the pool for service. If the pool cover is not removed on the scheduled service day than only chemical service will be provided that day. Windy/Rainy Days: 1. The water chemistry will be checked, necessary chemicals added, baskets emptied and large debris netted. This will constitute service for the week. It may take multiple service calls to return your pool and spa to its optimum condition after inclement weather. You may also ask to have a Special Clean done to immediately return you pool or spa to its optimum condition, however this will be done at an extra charge. Repairs: 1. 2. Minor repairs, such as the replacement of skimmer and pump baskets, skimmer weir gates and filter tank pressure gauges will be done automatically and billed to the customer as applied. Major repairs are discussed with the customer and approved before proceeding. Adding Water: 105 1. It is the responsibility of the customer to maintain the proper water level of the swimming pool and spa; however water may be added by the service technician at the time of service to help insure that proper water levels are maintained. Payment: 1. 2. 3. 4. 5. Monthly service is billed before the service month and payment is expected by the 20th of the service month. Chemicals will be billed, as per usage on the following month. Payment for any major repair work will be due upon completion. Any payment not received by the end of the month will be subject to a late fee of $15.00 or 5% of the amount owed, whichever is greater. After 2 months of non payment service will be suspended and 30, 60 and 90 day late payment and delinquency claims (as applied) may be reported to Equifax, Experian and TransUnion credit bureaus as well as any appropriate claims to the Better Business Bureau at the discretion of Acme Water Pool Service. Holidays 1. 2. 3. There are four months containing five weeks in that month in which there are no additional service fees. This in turn balances out the legal holidays or vacation time observed. The week prior to a holiday or vacation extra sanitizer will be added to your pool or spa. Acme Water Pool Service is closed and service is not performed on the following days: New Year’s Eve and Day, Easter, Memorial Day, Fourth of July, Thanksgiving, Christmas Eve and Christmas Day. 1 Week vacation may be taken at Easter, Thanksgiving and Christmas or as otherwise advised. Advanced notice will be given prior to such vacations. Weekly Pool Service: $___________ Per Month Bi-Annual Filter Sanitations: $___________ Each Acme Water Pool Service ~ 1234 Example Ave., Happytown, Ca 98765 ~ (818) 555-1234 Bus. Lic. # I-57302 ~ Insured by Arrow Insurance ~ Water Chemistry Certified by I.P.S.S.A. since 1985 Acme Water Pool Service Quality Pool and Spa Service since 1985 Residential Spa Service Agreement Service Includes: 1. 2. 3. 4. 5. 6. 7. 8. Test water chemistry and add chemicals: chlorine, pH balance, total alkalinity, cyanuric acid, calcium hardness and phosphate levels. Clean tile, dirt and grease line only (calcium removal will be charged extra upon request). Net the top and bottom of leaves and debris. Vacuum the surface as needed. Brush the walls and surface as needed. Clean out the skimmer and pump baskets of debris. Inspect the equipment to insure that all is in working order. Backwash the filter as needed. Filter Sanitations: 1. 2. 3. Filters will be sanitized, inspected, cleaned and parts lubricated at a charge of $ 85.00 per filter for Diatomaceous and Cartridge filters. Filter sanitations are done once every 6 months. Sand filters can only be backwashed. To open a sand filter and stir the sand or replace the sand will be an extra repair charge. Spa Draining and Refill: 1. 2. 3. The spa will be drained and refilled every 6 months to ensure the health of the water. The home owner will be responsible for refilling the spa and contacting the technician once it is full to begin the chemical start-up. The cost to drain and chemically start-up the spa is $85.00. Windy/Rainy Days: 1. The water chemistry will be checked, necessary chemicals added, baskets emptied and large debris netted. This will constitute service for the week. It may take multiple service calls to return your pool and spa to its optimum condition after inclement weather. You may also ask to have a Special Clean done to immediately return you pool or spa to its optimum condition, however this will be done at an extra charge. Repairs: 1. 2. Minor repairs, such as the replacement of skimmer and pump baskets, skimmer weir gates and filter tank pressure gauges will be done automatically and billed to the customer as applied. Major repairs are discussed with the customer and approved before proceeding. Adding Water: 1. It is the responsibility of the customer to maintain the proper water level of the spa; however water may be added by the service technician at the time of service to help insure that proper water levels are maintained. Payment: 1. 2. 3. 4. Monthly service is billed before the service month and payment is expected by the 20th of the service month. Chemicals will be billed, as per usage on the following month. Payment for any major repair work will be due upon completion. Any payment not received by the end of the month will be subject to a late fee of $15.00 or 5% of the amount owed, whichever is greater. 106 5. After 2 months of non payment service will be suspended and 30, 60 and 90 day late payment and delinquency claims (as applied) may be reported to Equifax, Experian and TransUnion credit bureaus as well as any appropriate claims to the Better Business Bureau at the discretion of Acme Water Pool Service. Holidays 1. 2. 3. There are four months containing five weeks in that month in which there are no additional service fees. This in turn balances out the legal holidays or vacation time observed. The week prior to a holiday or vacation extra sanitizer will be added to your spa. Acme Water Pool Service is closed and service is not performed on the following days: New Year’s Eve and Day, Easter, Memorial Day, Fourth of July, Thanksgiving, Christmas Eve and Christmas Day. 1 Week vacation may be taken at Easter, Thanksgiving and Christmas or as otherwise advised. Advanced notice will be given prior to such vacations. Weekly Spa Service: $___________ Per Month Drain and Refill Spa: $___________ Every 6 Months Filter Sanitation: $___________ Every 6 Months Acme Water Pool Service ~ 1234 Example Ave., Happytown, Ca 98765 ~ (818) 555-1234 Bus. Lic. # I-57302 ~ Insured by Arrow Insurance ~ Water Chemistry Certified by I.P.S.S.A. since 1985 Acme Water Pool Service Quality Pool and Spa Service since 1985 Commercial Pool and Spa Service Agreement Service Includes: 1. 2. 3. 4. 5. 6. 7. 8. Test water chemistry: chlorine, pH balance, total alkalinity, cyanuric acid, calcium hardness and phosphate levels. Clean tile, dirt and grease line only (calcium removal will be charged extra upon request). Net the top and bottom of leaves and debris. Vacuum the surface as needed. Brush the walls and surface as needed. Clean out the skimmer and pump baskets of debris. Inspect the equipment to insure that all is in working order. Backwash the filter as needed. Filter Sanitations: 1. 2. 3. Filters will be sanitized, inspected, cleaned and parts lubricated as needed at a charge of $ 85.00 per filter for Diatomaceous and Cartridge filters. On the average this service is done once every 3 to 6 months depending upon the filter pressure and usage of the swimming pool or spa. Sand filters can only be backwashed. To open a sand filter and stir the sand or replace the sand will be an extra repair charge. Windy/Rainy Days: 1. The water chemistry will be checked, necessary chemicals added, baskets emptied and large debris netted. This will constitute service for the week. It may take multiple service calls to return your pool and spa to its optimum condition after inclement weather. You may also ask to have a Special Clean done to immediately return you pool or spa to its optimum condition, however this will be done at an extra charge. Repairs: 1. 2. Minor repairs, such as the replacement of skimmer and pump baskets, skimmer weir gates and filter tank pressure gauges will be done automatically and billed to the customer as applied. Major repairs are discussed with the customer and approved before proceeding. Adding Water: 1. It is the responsibility of the customer to maintain the proper water level of the swimming pool and spa; however water will be added by the service technician at the time of service to help insure that proper water levels are maintained. Payment: 1. 2. 3. 4. 5. Monthly service is billed before the service month and payment is expected by the 20th of the service month. Chemicals will be billed, as per usage on the following month. Payment for any major repair work will be due upon completion. Any payment not received by the end of the month will be subject to a late fee of $15.00 or 5% of the amount owed, whichever is greater. After 2 months of non payment service will be suspended and 30, 60 and 90 day late payment and delinquency claims (as applied) may be reported to Equifax, Experian and TransUnion credit bureaus as well as any appropriate claims to the Better Business Bureau at the discretion of Acme Water Pool Service. Holidays 107 1. 2. 3. There are four months containing five weeks in that month in which there are no additional service fees. This in turn balances out the legal holidays or vacation time observed. The week prior to a holiday or vacation extra sanitizer will be added to your pool or spa. Acme Water Pool Service is closed and service is not performed on the following days: New Year’s Eve and Day, Easter, Memorial Day, Fourth of July, Thanksgiving, Christmas Eve and Christmas Day. 1 Week vacation may be taken at Easter, Thanksgiving and Christmas or as otherwise advised. Advanced notice will be given prior to such vacations. Pool #1: $___________ x _______ Services Per Week = $_____________ Per Month Pool #2: $___________ x _______ Services Per Week = $_____________ Per Month Spa #1: $___________ x _______ Services Per Week = $_____________ Per Month Spa #2: $___________ x _______ Services Per Week = $_____________ Per Month Other $___________ x _______ Services Per Week = $_____________ Per Month Chemicals Billed Separately Liquid Chlorine per gallon = Calcium Hypochlorite per lbs. = Dichlor Granular per lbs. = 3 inch Chlorine Tablets, each = Muriatic Acid per gallon = Potassium Monopersulphate per lbs. = Sodium Bromide per oz. = Conditioner per lbs. = Phosphate Treatment, per = $ 3.75 $ 3.00 $ 3.65 $ 1.50 $ 3.85 $ 4.00 $ 0.50 $ 2.50 $ 45.00 Monthly Total = $_____________ Per Month Acme Water Pool Service ~ 1234 Example Ave., Happytown, Ca 98765 ~ (818) 555-1234 Bus. Lic. # I-57302 ~ Insured by Arrow Insurance ~ Water Chemistry Certified by I.P.S.S.A. since 1985 Acme Water Pool Service Quality Pool and Spa Service since 1985 Wind and Rain Advisory During the wind and rain season your service technician may be limited in service. If it is raining or the wind is blowing you will still receive service, however it may be limited to only maintaining your pool/spa’s safety rather than the cosmetics of it. Your pool/spa may require several hours of work to recover from weather damage your technician can only spend up to 30 minutes maintaining it to insure that all customers are serviced. Ultimately, while your technician is there once a week the home owner still owns the pool/spa and is ultimately responsible for its safety and wellbeing. The chemicals will be balanced, the skimmer and pump baskets will be checked and emptied and any large debris that may cause damage to your pool/spa will be removed. Vacuuming and cosmetic service will wait until the following service, provided there are better weather conditions. There will also be heavy amounts of dirt in your pool/spa and your technician can only vacuum as well as your equipment will allow. If you have old or out dated equipment recovery can be a very slow process. If you require extra service to clean your pool/spa more quickly you can schedule an extra service call with your technician at a rate of $ 35.00 per 30 minute service call. Keep in mind that some weather damage clean up can take several hours. Here are a few things that you can do to help keep your pool/spa safe during the wind and rain season: Pull out any large debris that might cause damage to your pool/spa Check the skimmer and pump baskets and empty them frequently between service visits if they are full, else they will break and cause damage to your pump Check your floor cleaner if you have one for any clogs or stuck debris to help keep it running properly 108 If in doubt and you fear damage may occur, turn your pool/spa timer off and contact your service technician Remove any glass tables from the pool/spa area during the windy season as it is in risk of knocking over, breaking and glass and other hazardous debris being knocked into the pool/spa Remember, when the winds and rain come everyone’s pool/spa are affected. It may take a few weeks to recover, however the more you are able to help with the weather damage clean up the faster your pool/spa will be safe and looking beautiful again. Acme Water Pool Service Quality Pool and Spa Service since 1985 Summer Health and Safety Your pool/spa can be the focal point of your back yard and a great source of relaxation and enjoyment. Owning a pool/spa is also a great responsibility. If health and safety are neglected a pool/spa can become a hazard and unsafe liability of your home. The following is a list of helpful tips that can help minimize any health and safety risks associated with your pool/spa and can help to maximize your enjoyment of it. 109 1. Make sure your pool/spa water is maintained at the proper level. Your pool/spa should always be filled to at least half way up the skimmer opening (the rectangular box opening along the top of the pool/spa). Low pool/spa water can result in air being sucked into the pump causing it to run dry and burn out. Normal evaporation can result in your pool/spa losing as much as 2 or more inches of water per week. Be sure to check your pool/spas water level at least twice per week and after each use. 2. Rinsing your face and body off before swimming will greatly reduce any redness of the eyes, itchiness and other swimmer discomforts commonly associated with chlorine. Chlorine bonds with germs, bacteria and swimmer wastes such as sweat, dead skin, mucous and tears creating chloramines (that bad chlorine smell created when chlorine bonds with and begins to burn away waste). Chloramines are really what cause most swimmer discomfort, not the chlorine itself. Rinsing off before swimming will remove any swimmer waste and prevent uncomfortable chloramines from forming. 3. Make sure all pool/spa area and related gates are working properly. Keeping your pool/spa secure from unattended children can help to prevent accidents and drowning. 4. Remove all toys from the pool/spa area after use. Toys in the pool/spa and surrounding area can lure small unattended children risking accidents and drowning. 5. Remove large debris from pool/spa between service visits. Often, during the windy season the strong winds can knock chairs, tables, tree branches and other large debris into your pool/spa and such debris left there can cause damage. 6. Check your pool/spas skimmer and pump baskets between visits during the windy season. Depending on the amount of debris around the pool/spa area the winds can knock a large amount of debris into your pool/spa. Much of this debris will be collected in your skimmer and pump baskets. If these baskets become full from too much debris they can break allowing that debris to clog or damage your pump. Keep the baskets clean between service visits during the windy season to help reduce any risks of damage. 7. Do not run around the pool/spa area. The area around the pool/spa can become slippery from the water and running on a wet surface can result in accidents and injury. 8. Protect yourself from the Sun’s harmful ultra violet rays. Too much exposure to the Sun’s ultra violet rays can result in sun burn or similar skin related conditions. Be sure and use proper protection. 9. Contact your service technician before having a pool/spa party so that he can prepare the water prior to the event for heavy usage and to leave with you with any extra needed chemicals to add after the party. Additional charges for extra chemicals may apply. Acme Water Pool Service Quality Pool and Spa Service since 1985 1 Month Free Service Since the founding of Acme Pool Service in 1997 the 1 month free service for references has applied. We have never advertised in any publication or otherwise. Rather than pay other services for advertising 110 we at Acme Pool Service would rather pay our customers back for their assistance in helping our business to grow. We offer 1 month of free service for any reference that signs up for monthly service. It is always an honor for a business to be referred to a customer’s friends, family and neighbors as it shows a satisfaction in quality of service. Please feel free to contact us or have any reference contact us and mention you as the reference to receive a free month of service. There is no limit to how many times this will apply to your account, although service is restricted to certain areas within our route coverage. Thank you again sincerely for your business and support. Sincerely, Acme Water Pool Service Acme Water Pool Service Quality Pool and Spa Service since 1985 2008 Vacation List The following is a list of vacation days taken by your technician for 2008. These scheduled annual vacation days will not be reflected in your monthly bill. If you are in need of service during these vacation days please contact your technician to schedule an extra service call. 111 Holiday New Year’s Day 2008 Spring Vacation Memorial Day Independence Day Labor Day Veterans/Patriots Day Thanksgiving Winter Vacation New Year’s Day 2009 Days Tue. Jan. 1st, 2008 Mon. Mar. 24th thru Sun. Mar. 30th Mon. May 26th Fri. July 4th Mon. Sept. 1st Thur. Sept. 11th Thur. Nov. 27th and Fri. Sept. 28th Thur. Dec. 25th thru Wed. Dec. 31st Thur. Jan. 1st, 2009 Acme Water Pool Service 123 N. Imaginary Ave. Happy Town, Ca 98765 Invoice # Payment Due Date 1108 11/20/08 Annual Account Summary (818) xxx-xxxx Monthly Service Conditioner (Feb. and Sept.) Algaecide (June) Filter Sanitation (Apr. and Oct.) Joe Schmoe 1234 E. Customer Ln. Coolsville, Ca 91234 112 $ 95.00 $ 35.00 $ 75.00 $ 85.00 Quantity Item/Service Price October service Filter sanitation Filter tank o-ring $ 95.00 $ 85.00 $ 12.50 1 Thank you for your business. Total Due $ 192.50 Please make check payable to: Acme Water Pool Service ----------------------------------------------------------------------------------------Please note: A $15.00 late fee will be applied to any payment not Helpful Tips of the Month received by the 30th of each month. Invoice # Payment Due Date Total Due Payment Enclosed * These dry air conditions will cause evaporation of your swimming pool water. Be sure and check your water level and add water regularly. 1108 11/20/08 $ 192.50 * November holidays: Thurs and Fri, November 27th and 28th Thanksgiving. No service provided on those days. Payment From: Joe Schmoe 1234 E. Customer Ln. Coolsville, Ca 91234 * The Santa Ana wind season is here. Please keep any glass table tops away from the pool area and cut back any excessive foliage if able. Keep pump and skimmer baskets clean. Acme Water Pool Service Quality Pool and Spa Service since 1985 Field Technician Water Chemistry Analysis Date: _______ Pool Type: Spa Type: O O O O Customer: ________________ Technician: ______________ Plaster Fiberglass Plaster Fiberglass O O O O 3M Color Quartz Vinyl 3M Color Quartz Vinyl O O O O Painted Pebble Tech Painted Pebble Tech Pool/Spa Size: ________ Gallons Pool/Spa Surface Conditions: _________ 113 Present Condition of Water: Type of Filtration: Clear _______ Cloudy______ Algae______ D.E._________ Cartridge_________ Sand___________ Filter Pressure: ______PSI Type of Chlorination: Salt______ 3” Tab Floater______ Chlorinator__________ Water Test Results Test Result Ideal Range Free Available Chlorine Bromine pH Balance Total Alkalinity Cyanuric Acid (Conditioner) Total Dissolved Solids Calcium Hardness Copper/Iron Phosphates Salinity (Salt) 1. 2. 3. 4. 5. 6. 7. 8. 3.0 - 5.0 ppm 3.0 - 5.0 ppm 7.4 - 7.6 80 - 120 ppm 70 - 90 ppm 2500 ppm Max 200 - 500 ppm 0.2 ppm Max 0 - 200 ppm Max 3000 - 3500 ppm Notes ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ ___________________________________________________________ Pool Draining, Tile Cleaning, Acid Wash & Repair Waiver Disclosure Statement And Acknowledgement DRAINING AND/OR ACID WASH TREATMENT OF A SWIMMING POOL’S FINISH ARE MAINTENANCE PROCEDURES USUALLY NEEDED PERIODICALLY THROUGHOUT THE LIFE OF A SWIMMING POOL. HOWEVER, SINCE THESE PROCEDURES INVOLVE DRAINING THE SWIMMING POOL AND APPLYING CHEMICALS TO THE PLASTER, THERE ARE CERTAIN RISKS INVOLVED NOT NORMALLY ASSOCIAED WITH DAY TO DAY MAINTENANCE PROCEDURES AND WHICH ARE NOT WITHIN THE CONTROL OF THE POOL SERVICE TECHNICIAN. THE PURPOSE OF THIS STATEMENT IS TO PROVIDE YOU, THE CUSTOMER, WITH ADDITIONAL INFORMATION CONCERNING THESE PROCEDURES AND INFORM YOU OF SOME OF THE RISKS INVOLVED AND WHAT THE TYPICAL RESULTS OF SUCH PROCEDURES ARE. Periodic draining of swimming pool water is a common maintenance practice. It is routinely performed to remove water that has become hard or laden with excessive minerals, or to perform repairs to a pool. Normally, removal of water from a pool causes no problems. However there are a few things that can happen of which you should be aware. When the water is removed, the pool may rise out of the ground, a condition often times caused by hydrostatic pressure (i.e., too much moisture in the soil). Once exposed to air, tile may fall off the pool; the plaster can shrink, expand, crack, blister, flake or pop off, etc. These problems do not normally occur, and are beyond the control of the person who has simply "drained the water." However, the possibility of these problems can be reduced by not draining the pool during the wetter times of the year, not leaving the pool empty for more than 48 hours during hot or dry weather before refilling. Repairs that require the draining of a pool should be made as quickly as possible and the pool refilled as soon as possible. 114 ______________________________________ will empty your pool/spa, for the following service needs: __________________________________________________________________________________________________________________ Owner acknowledges that _______________________________________________ is not responsible for the structural integrity of an empty pool/spa. Any and all damages, losses, and expenses, including, but not limited to structural problems, existing or new movement, water table conditions, soil conditions or acts of nature that contribute to structural damage to the swimming pool/spa, coping, decking, tile, plumbing, electrical, or related surface failure of (plaster, fiberglass, pebble or paint), arising from empty pool/spa is the responsibility of the owner. WARNING: Special problems to plaster, fiberglass or other pool surfaces, such as crazing, spalling, chipping, peeling or de-lamination may result from emptying the pool/spa. These conditions may also occur during the refill, when performing the needed repairs, or after the completion of the repairs. It is fully understood that if these problems do occur it is beyond the control of responsibility of the pool service company. The pool/spa should be left empty only to complete repairs in a timely manner. The owner is advised to contact the installation contractor (plaster or fiberglass) prior to emptying the pool/spa. Several instructions or warranty issues may need clarification prior to emptying the pool/spa. ACID WASHING AND ACID TREATMENTS The decision to use acid procedures to remove stains and mineral build-up from a pool’s surface should be very carefully considered. Under most circumstances staining or mineral build-up takes many months or years to accumulate. While acid treatments are recognized as a common procedure for removal, there are several problems that may occur. The process of applying acid to plaster surfaces may cause the surface to etch, become rough or expose the aggregate in the plaster mix. To what degree this occurs depends on the concentration of acid, the temperature of the stain being removed and the quality and condition of the plaster itself. In some cases cracking, thinning or delamination of the tile and plaster could be a pre-existing condition and is beyond the control of the acid wash applicator. Consideration should be given to the experience and recommendation of the applicator and if you have any doubts, seek a second opinion and/or additional information. An evenly colored, smooth texture after an acid procedure is a totally unrealistic expectation. The stains most likely took a long period of time to develop, and could be embedded deeply into the plaster material. At best, the consumer can expect the pool’s appearance to look "brighter" than before, with some stains remaining. Sanding will aid in the restoration of the pool’s surface texture. BY SIGNING IN THE PLACE INDICATED BELOW YOU ARE ACKNOWLEDGING THAT YOU HAVE READ AND UNDERSTOOD THE RISKS, TERMS, CONDITIONS AND OTHER INFORMATION DISCLOSED THAT THE ABOVE LISTED. YOU HAVE WAIVED ALL RIGHTS TO DAMAGE DONE TO THE POOL/SPA AS A RESULT FROM REMOVING THE WATER FROM THE POOL/SPA AND THAT COMPLETE REMOVAL OF ALL STAINS IS NEITHER GUARANTEED NOR REPRESENTED. Owner’s name_____________________________ Owner’s Signature___________________________ Address: ________________________________________________________Date: __________________________ 26. Service Technician Training Guide Tests These tests are here to reflect on the lessons you have learned in this training manual. Hopefully this manual has proven to be a quality guide to help you along in becoming a knowledgeable and quality swimming pool service technician. Section 3: Three Keys to Clear, Clean and Healthy Water: 1. There are 3 keys to clear, clean and healthy water. They are: O A. Chlorine, acid and salt 115 O B. Clarity, fluidity and taste O C. Circulation, filtration and chemical balance 2. Circulation is: O A. The flow or current in water O B. How the water is filtered O C. When you super chlorinate or shock the water 3. An average sized pool and spa of about 20,000 gallons should circulate for how long per day? O A. All day long O B. 4 – 5 hours per day O C. Once per week O D. 6 – 8 hours per day 4. When a body of water is not circulating it becomes: O A. Sun damaged O B. Bad smelling O C. Stagnant 5. There are three primary types of filters in the swimming pool service. They are: O A. Metal, plastic and fiberglass O B. Silver, tan and grey O C. Diatomaceous earth, cartridge and sand 6. The entire body of water should be passed through the filter at least how often? O A. Once per day O B. Once per week O C. Once per month 7. The filter’s job is to hold what sort of material? O A. Water, chlorine and acid O B. Earth, wind and fire O C. Dirt and debris 8. What will cause dirt and debris to pass through the filter and back into the swimming pool? 116 O A. An improperly assembled filter O B. A tear in one or more of the filter grids or cartridge O C. Damage to the inner structure of the filter O D. All of the above 9. What is the single most important key to keeping water healthy and killing algae? O A. Circulation O B. Filtration O C. Chemical balance 10. Bad water chemistry can cause what kinds of problems? O A. Swimmer discomfort O B. Swimmer illness O C. Damage to the surface O D. Damage to the equipment O E. All of the above Section 4: About Chlorine, Sanitizers and Oxidizers: 1. Chlorine is a Greek word meaning: O A. Khlôros, meaning 'pale green' O B. Sulfuric salt O C. White plastic bottle 2. Chlorine in its natural form is a: O A. Liquid O B. Solid O C. Gas 3. Chlorine is both a sanitizer and an oxidizer. What does it mean to sanitize? O A. Sanitize is to wash ones hands O B. Sanitize means to add soap O C. Sanitize means to kill germs and bacteria O D. Sanitize means to make clean 117 4. Chlorine is both a sanitizer and an oxidizer. What does it mean to oxidize? O A. To oxidize means to use oxygen O B. To oxidize, much like a chemical fire, means to burn up organic material including germs, bacteria, human waste such as sweat, dead skins cells, tears, mucous, urine and feces as well as other harmful organisms such as algae. O C. To oxidize means to add acid 5. When chlorine bonds with and begins to sanitize and oxidize organic material in the water it creates a foul chlorine and ammonia like smell. This combined chlorine is called a: O A. Chloramine O B. Hypochlorous acid O C. Water salinity 6. Chloramines are combined chlorine with organic material in the water. Chlorine bonds and burns away organic material such as human waste like sweat, dead skin cells, mucous, urine, feces and in this process causes a foul smell. Swimmers can eliminate this reaction by simply doing what before they enter the water? O A. Rinse off before swimming to eliminate the organic material that chlorine will bond with O B. Improve their personal hygiene and think about using deodorant now and again O C. brush their teeth more than once a day 7. Chloramines can cause what swimmer discomforts? O A. Irritable bowel syndrome O B. Bubonic plague O C. Red and irritated eyes and rashes and itchy, dry skin 8. What causes water to become dull, listless and cloudy? O A. Organic material in the water O B. A cloudy day O C. Kids peeing too much in the water O D. Dogs swimming 9. Liquid chlorine has a very high pH balance of about 13. To account for this pH increase for gallon of liquid chlorine added you should also add: O A. ¼ gallon of Muriatic acid O B. 1 gallon of 2% low fat milk 118 O C. Add more water to the pool 10. Dichlor granular chlorine has 2 elements in it. They are: O A. Chlorine and acid O B. Liquid chlorine and water O C. Hopes and dreams O D. Chlorine and conditioner 11. Trichlor 3” tablets have 3 elements in them. They are: O A. Chlorine, bromine and ammonia O B. Chlorine, acid and conditioner O C. Hopes, dreams and good intentions 12. Trichlor, whether it be in tablets or granular has acid in it and if left on the surface of a pool could cause what sort of damage? O A. The acid in the tablets or granular could etch the plaster or surface or even burn holes and stain the surface. Such damage is often seen on the top step of a plaster pool as the results of a 3” tablet floater sitting over the top step for long periods of time. O B. The acid could release air bubbles into the water O C. The acid could increase the filter pressure 13. 3” Trichlor chlorine tablets dissolve slowly, depending upon the temperature of the water and the circulation. While this form of chlorine does not add an immediate dose of chlorine to the water it can be: O A. An effective back up chlorination method to replace the chlorine used during heavy Use O B. An effective way to add conditioner to the pool O C. An effective way to lower the pH balance of the water 14. The negative effects of using Calcium Hypochlorite are: O A. It smells funny O B. It increases the calcium hardness due to the high calcium content and can temporarily cloud the water when added O C. Can increase the amount of conditioner in the water 15. Salt is Sodium Chloride. As the salt crystals pass through a salt system cell some of the chloride gas is freed from its confining sodium chloride crystals. Once free from the crystals the chloride gas by itself is called: O A. Salty O B. Chlorine 119 O C. Acid 16. The salt level for a salt chlorination system should be maintained at what levels? O A. 2000 – 5000 ppm O B. 3250 – 3500 ppm O C. 150 – 300 ppm 17. The chlorine generated from a salt chlorination system has a very high pH balance of about 13. To lower this pH balance and keep the chlorine in its effective killing range you must add what to the water? O A. Muriatic acid O B. More salt O C. Conditioner 18. Chlorine is only in its killing form in low pH balance conditions. What is the proper pH balance to maintain at all times for the most comfortable water for the swimmer and to keep the chlorine in its optimal killing form? O A. 6.5 – 7.0 pH O B. 7.4 – 7.6 pH O C. 8.0 – 8.2 pH 19. Chlorine is consumed by bonding with germs, bacteria and organic material but is destroyed by the Sun’s harmful ultra violet rays. What is added to the water to protect the chlorine from these U.V. rays? O A. Muriatic acid O B. Conditioner (Cyanuric acid) O C. Salt 20. Chlorine levels should be maintained at what level at all times? O A. 1.5 – 2 ppm O B. 6 – 10 ppm O C. 3 – 5 ppm Section 5: pH Balance (Power of Hydrogen): 1. pH is a measurement of: O A. How much chlorine is in the water 120 O B. The measure of the acidity or alkalinity of a solution O C. The amount of conditioner in the water 2. What does pH stand for? O A. Potens Hydrogen (Latin for Hydrogen Power) O B. Power of Hydrogen O C. Potential of Hydrogen O D. Pondus Hydrogenii (Latin) O E. Pouvoir Hydrogène (French) O F. All of the above 3. Chlorine is most active and in its killing form in low pH conditions. The ideal pH balance of swimming pool water should be: O A. 7.8 – 8.0 O B. 7.4 – 7.6 O C. 6.5 – 7.0 4. Who first introduced the concept of pH? O A. Danish chemist Søren Peder Lauritz Sørensen at the Carlsberg Laboratory in 1909 O B. Richard Nixon in Yorba Linda, Ca in 1978 O C. Charlie Taylor in St. Paul, Minnesota in 1983 5. One reason to keep the pH balance at 7.3 – 7.4 is because it is close to a human’s pH balance. What is a human’s natural pH balance? O A. 7.25 – 7.5 O B. 8.0 – 8.45 O C. 7.34 – 7.45 6. A pH below 7.0 causes water to become: O A. Lemonade O B. Acidic and corrosive, causing metals to dissolve and cause staining O C. Basic and alkaline 7. High pH balance can cause what harmful effects? O A. Scaling water O B. Plugged filters O C. Reduced circulation 121 O D. Cloudy water O E. Chlorine inefficiency O F. Swimmer irritation O G. All of the above 8. pH balance is lowered by adding: O A. Sodium Bicarbonate O B. Muriatic Acid O C. Liquid Chlorine 9. pH balance is increased by adding: O A. Soda Ash O B. Sodium Bromide O C. Conditioner 10. Water’s natural pH balance is: O A. 7.2 O B. 8.0 - 8.2 O C. 7.6 Section 6: Total Alkalinity (TA): 1. What is alkalinity a measurement of? O A. How much soda ash is in the water O B. The amount of acid in the water O C. The ability of a solution (water) to neutralize acids based on the amount of alkaline particles in the water 2. Total alkalinity is a measurement of the waters ability to neutralize: O A. Chlorine O B. Acid O C. Soda ash 3. Total alkalinity in swimming pool water should be maintained at: O A. 200 – 250 ppm O B. 80 – 120 ppm O C. 40 – 60 ppm 122 4. Alkaline particles determine the ability of water to resist changes in the: O A. pH O B. Free Available Chlorine O C. Calcium Hardness 5. What can be added to the water to increase the total alkalinity? O A. Muriatic acid O B. 3” Trichlor tablets O C. Sodium Bicarbonate (Baking Soda) 6. Total alkalinity and pH balance are both related in what way? O A. Muriatic acid lowers both of them when added O B. Total alkalinity determines the rate of change of pH balance O C. Total alkalinity acts as a buffer to protect pH balance from making drastic changes O D. All of the above 7. What is used to lower total alkalinity? O A. Liquid chlorine O B. Salt O C. Muriatic acid 8. If the total alkalinity is too low than when acid is added it will result in: O A. Drastic pH bounce and instability O B. Highly unbalanced water condition resulting in damage to copper heat exchangers, light rings, stainless steel ladders, and concrete pool surfaces O C. Both A and B 9. A high total alkalinity can result in: O A. Scaling water O B. Plugged filters O C. Reduced circulation O D. Cloudy water O E. Increased rise in pH balance O F. All of the above 10. While pH balance can change from one day to the next, total alkalinity is much slower to rise 123 and fall taking: O A. hours to change, rather than days O B. weeks to change, rather than days O C. years to change, rather than days Section 7: Conditioner, Stabilizer (Cyanuric Acid): 1. Cyanuric acid is also called and known as: O A. Conditioner O B. Stabilizer O C. Both A and B 2. Conditioner is added to the water slowly through the skimmer to: O A. Increase the pH balance O B. Clean the filter O C. Protect the chlorine from the Sun’s harmful ultra violet rays 3. Conditioner fall out staining will most often happen with older water and at very cold temperatures, though has been known to fall out in other conditions as well, such as low pH conditions from acidic water (below 7.0). What do conditioner stains look like? O A. At 110+ ppm you’ll see a light purple dust coat on the surface that can usually be brushed away. O B. At 120+ ppm you will see a heavier purple dust coat on the surface that does not brush away and the beginnings of a deeper shade of purple vertical lines, as if scribbled in with a Crayon, along the walls O C. At 130+ ppm you will see a deep purple dust coat and splotches on the surface as well as dark purple vertical lines, as if gouged in with a Crayon O D. All of the above 4. Without conditioner, chlorine will: O A. Be unprotected from the Sun’s U.V. rays and will not last long in the water, causing unstable chlorine bounce and allowing chlorine resistant algae to grow O B. Be increased, causing super chlorination or break point chlorination O C. Be in its maximum killing form 5. Too much conditioner in the water (over 100 ppm) can cause: O A. A light purple staining over the pool’s surface, plastic parts, baskets, floor cleaner, main drain lids and plumbing 124 O B. Chlorine instability O C. High Total Alkalinity 6. Name two types of chlorine that also have conditioner in them: O A. Muriatic Acid and Liquid Chlorine O B. Calcium Hypochlorite and Sodium bromine O C. Dichlor granular chlorine and Trichlor chlorine including granular, 1 inch and 3 inch tablets 7. How do you reduce the conditioner level in a pool if the conditioner level is too high? O A. Add more chlorine to use up the conditioner O B. Back washing the filter, splash and draining the water out of the pool O C. Add muriatic acid 8. Conditioner is never added directly to the pool as it is an acid and when sitting on the surface can etch and stain the surface. Conditioner also has to break down under pressure. How do you apply conditioner to the pool water? O A. Just add Dichlor and 3 “ Trichlor tablets only to slowly raise the conditioner level O B. Dump it all in the skimmer at one time and turn off the pump O C. With the pump running, slowly add the conditioner, 1 to 2 lbs at a time in through skimmer, wait a few minutes, and add more as needed as to not clog up the pump basket. Make sure the pump runs for at least 4 hours to break down the conditioner inside the filter under pressure to slowly disperse it into the swimming pool water. 9. Why should you never try to raise the conditioner level to 90 to 100 ppm all at one time? O A. You risk over estimating how much conditioner is needed and add too much and have to drain the pool down to get rid of the excess conditioner O B. Conditioner is expensive and you want to save some for later O C. Too much conditioner will cause you to lose chlorine in the water 10. The conditioner level should always be kept at: O A. 40 – 60 ppm O B. 100 – 150 ppm O C. 70 – 90 ppm Section 8: Calcium Hardness: 1. Hard water is the type of water that has high: 125 O A. Conditioner O B. Chlorine levels O C. Mineral content 2. Hard water minerals primarily consist of: O A. Copper and Iron O B. Calcium (Ca2+), and Magnesium (Mg2+) metal cations O C. Cobalt and Manganese 3. The term “hard” water comes from what industry? O A. The term “hard” comes from the laundry detergent industry and means that it is “hard” to form suds O B. The term “hard” water comes from the swimming pool industry and means the water is “hard” to kill algae O C. The term “hard” water comes from the chlorine industry and means the water is “hard” to retain chlorine 4. Water with calcium hardness below 180 ppm will become: O A. Corrosive and look to pull calcium out of the plaster, tile grout and the concrete in the deck O B. “Hard” and should be drained O C. Ideal and should be kept below 180 ppm 5. Water with high calcium hardness, over 500 ppm: O A. Scaling water and plugged filters O B. Reduced circulation and cloudy water O C. Heater inefficiency and additional calcium deposits on the tile and spill ways O D. All of the above 6. Calcium hardness, when below 180 ppm, can be raised by adding: O A. Muriatic acid O B. Sodium bicarbonate O C. Calcium elevator 7. You can reduce the calcium hardness in the water by: O A. Add more chlorine to neutralize the calcium O B. Back washing the filter, splash and draining the water out of the pool 126 O C. Add soap to the water 8. Calcium hardness should be maintained at: O A. 180 to 500 ppm O B. 100 to 150 ppm O C. 500 to 1,000+ ppm 9. According to the United States Geological Survey, what percent of homes in the U.S. have hard water? O A. 62.8% O B. 25.5% O C. 89.3% 10. Hard water leaves the swimmer’s skin feeling “less than clean”, while soft water leaves the swimmer’s skin feeling: O A. Dry and irritated O B. Slippery O C. Like there is a film or debris sticking to it Section 9: Algae! Know Your Enemy: 1. Algae are: O A. Single-cell plants containing chlorophyll. They are some of the hardiest and most widespread organisms living on the planet, existing in over 30,000 different varieties O B. Discolorations in the plaster resulting from bad water chemistry O C. Stains left from leaves and debris 2. What does algae require to grow? O A. Warm water O B. Sunlight O C. Carbon Dioxide O D. All of the above 3. Yellow algae can exist and grow even in water with how much chlorine? O A. 0.5 to 3.0 127 O B. 1.0 to 3.0 O C. 3.0 to 5.0 O D. All of the above 4. Yellow algae appear as: O A. A yellow powdery growth O B. A green thick growth covering the entire pool O C. Small bluish-black spots 5. There are about how many species of green algae? O A. 600 O B. 6000 O C. 60 6. The green algae (singular: green alga) are the large group of algae from which the embryophytes (higher plants) emerged. This means that green algae is one of the primary sources that other plants have eveolved from. Is this statement true or false? O A. True O B. False 7. Green algae can be found: O A. On the walls O B. On the floor O C. Floating in the water, giving it that green tinge O D. Floating on the surface O E. All of the above 8. Cyanobacteria, also known as blue-green algae, blue-green bacteria or Cyanophyta, is a phylum of bacteria that obtain their energy through photosynthesis. The name "cyanobacteria" comes from Greek word κυανός (kyanós) for the color of the bacteria and means: O A. Black O B. Blue O C. Bacteria 9. Stromatolites of fossilized oxygen-producing cyanobacteria have been found from: 128 O A. 280 million years ago O B. 2.8 million years ago O C. 2.8 billion years ago 10. Black (blue-green) Algae is evident by the formation of: O A. Yellow powdery deposits on the walls O B. Small dime (or smaller) to quarter sized black (or blue-green) spots O C. A green tinge to the water 11. Black (blue-green) algae is: O A. Present by thick, black slimy nodules tenaciously adhering to the surface O B. Very resistant to chlorine O C. Likely to return over time due to having very deep roots O D. All of the above 12. Algae can bloom: O A. Within 12 hours in untreated water O B. When the pH balance rises causing the chlorine to become inefficient O C. When the conditioner level is too low causing chlorine bounce or unstable chlorine levels O D. All of the above 13. Phosphates are: O A. Microscopic dead plant material that provide food for algae O B. Found in almost everything, including the tap water, air, plants and even within our own body O C. Absorbed by algae as a food source and once the algae blooms it has consumed all the phosphates it needs to live out its entire life O D. All of the above 14. Algae is harmful and unhealthy for swimmers because: O A. It harbors and protects germs and bacteria O B. It looks unsightly O C. It sticks to the swimmer 15. Algae can grow, all though it would be weak and unhealthy, even in perfect water chemistry conditions: O A. True 129 O B. False Section 10: Algaecides and Phosphate Removers: 1. There are three main groups of algaecides: O A. Copper, ammonia and sodium bromine O B. Silver, copper and zinc O C. Quats, polyquats and copper salts 2. An algaestat is: O A. A preventative maintenance treatment to prevent algae growth O B. A powerful algaecide that kills algae on location O C. A mixture of copper and bromine 3. Metal based algaecides cause the chlorine to work more efficiently, however can also cause: O A. Rapid chlorine depletion O B. Staining on the swimming pool surface, equipment and tile O C. The algae to become resistant or immune to chlorine 4. Liquid application, zinc balls and Nature2 cartridges are all ways of distributing: O A. Chlorine evenly throughout the water O B. Metals into the water O C. Sanitizer into the water 5. Many metal based algaecides, such as the Nature2 system claim that you can allow the chlorine level to drop to as low as 1.5 ppm (parts per million) due to the chlorine being more efficient because of their product, however without enough sanitizer and oxidizer in the water to kill germs and bacteria and burn away organic material water will become: O A. Dull and cloudy O B. Tinged in a green color O C. Drinkable and healthy 6. Sodium Bromine works just as chlorine does as a sanitizer and an oxidizer however, it is 130 different in that: O A. It has metals in it O B. It is unaffected by pH balance and is not protected by conditioner O C. It has acid in it 7. As sodium bromine is destroyed by the Sun’s U.V. rays chlorine will: O A. Sacrifice itself to recharge the sodium bromine O B. Take the place of sodium bromine as a sanitizer and oxidizer O C. Further speed up the destruction process as to remove the sodium bromine 8. When using a sodium bromine based algaecide it is good to: O A. Add additional chlorine to keep the sodium bromine charged O B. Lower the pH balance to help the sodium bromine sanitize more effectively O C. Raise the conditioner level to protect the sodium bromine 9. Phosphates are: O A. Algae spores O B. Dead plant material and food for algae O C. Plants that fall into the water 10. Once algae blooms it has consumed enough phosphates to: O A. Live its entire life O B. Destroy the chlorine in the water O C. Neutralize the acid in water 11. Phosphates can be found in: O A. Drinking water, food and the human body O B. Air, soil and swimming pool fill water O C. Liquid chlorine and many other swimming pool service chemicals O D. All of the above 12. Algae need very little phosphates in order to bloom, colonize and live its entire life: O A. True 131 O B. False 13. When algae die it becomes: O A. Chloramines O B. A sanitizer and oxidizer O C. Phosphates 14. Phosphate removers work by: O A. Causing the phosphates in the water to clump up together in large enough amounts to be collected in the filter O B. Sanitizing and oxidizing the phosphates O C. Politely asking the phosphates to relocate to someone else’s pool 15. When phosphates clump up together they can create more mass and fall out of circulation causing a white, slimy snow like fall out on the surface. When this is vacuumed up or makes its way to the filter the filter pressure will: O A. Greatly increase and the filter will need to be backwashed and possibly even cleaned O B. Remain the same and compact the phosphates O C. Automatically dispose of the phosphates 16. Algae can bloom when: O A. The sanitizer and oxidizer (chlorine usually) become too low O B. The pH balance becomes too high and thus not allowing the sanitizer to work O C. The conditioner level is too low causing the chlorine level to radically bounce and be unstable O D. At anytime, even in perfect water chemistry conditions O E. All of the above 17. Chlorine is poisonous to algae, however if the chlorine is weak due to high pH, is unstable due to low conditioner levels or the amount of regular Free Available Chlorine is not maintained at a high enough level than: O A. The algae can not bloom O B. The muriatic acid will be ineffective O C. The algae may be weakened, but not killed and as it colonizes and continues to grow it becomes very resistant to chlorine 18. Always brush algae with a wall brush when treating it to: 132 O A. Knock away the protective blossom (the part of algae that we can see) to expose the roots to the swimming pool water’s chemicals and algaecides O B. Remove the visible algae, making the pool surface look better O C. Both A and B 19. After an algae removal has been done it is good to backwash or clean the filter to: O A. Create another charge for the customer O B. Remove conditioner from the water O C. Remove the dead algae from the filter and maximize circulation and filtration 20. When scrubbing black algae with a steel wire brush it is best to: O A. Scrub lightly as to not damage your brush O B. Only scrub the places where you can see the algae O C. Rinse your brush off afterwards with liquid chlorine as black algae can be transmitted from one pool to another Section 12: Salt Chlorination Systems: 1. Salt is composed of: O A. Muriatic acid O B. Sodium chloride or sodium and chlorine O C. Chlorine, acid and conditioner 2. The salt chlorination system’s cell frees the: O A. Chlorine from the sodium chloride crystals O B. Acid from the hypochlorous acid O C. Salt from the water 3. The chlorine freed and introduced to the water is in its gas form and has a high pH balance of: O A. 11 to 13 O B. 6.5 to 8.0 O C. 7.0 4. Because salt systems constantly increase the pH balance of the water, and being that chlorine needs low pH conditions to be in its killing form more acid than normal must be used to keep the pH balance at: 133 O A. 8.0 to 8.2 O B. 7.6 to 7.8 O C. 7.4 to 7.6 5. A salt cell should be inspected and if need be cleaned once every: O A. 90 days O B. Year O C. Month 6. The main benefit to the swimmer of using a salt chlorination system is that: O A. A salt chlorination system is much less expensive to operate than traditional chlorine methods O B. The water requires far less acid to balance the pH O C. The water feels softer and easier on the swimmer 7. By adding more acid to reduce the pH balance to a salt chlorinated pool than a regular pool, not only the pH is being lowered but also the: O A. Total Alkalinity O B. Conditioner level O C. Calcium Hardness 8. Most salt chlorination systems require the salt level to be between: O A. 4,000 to 5,000 ppm O B. 1,000 to 1,500 ppm O C. 3,250 to 3,500 ppm 9. Salt is a natural corrosive to metals and can rust through and destroy a metal filter: O A. True O B. False 10. It is always important to test the salt level independently, separately from the salt system computer board using a salt test strip or an electronic salt reader because: O A. It is less expensive O B. The computer board can easily and often become inaccurate and give false readings O C. It is faster 134 Section 13: About Swimming Pool Filters: 1. Most diatomaceous earth filters use how many grids?: O A. 6 O B. 4 O C. 8 2. If there is a tear in the filter grid of a D.E. filter: O A. The water will lose chlorine more rapidly O B. The dirt and debris will leak through the tear and back into the pool O C. The filter will collapse and you will need to replace the entire filter 3. When in backwash mode a D.E. filter will send much, but not all of the dirt and debris: O A. Into the sewer line if it was properly plumbed or simply out through the backwash line O B. Back into the swimming pool O C. Into the spa 4. How much Diatomaceous Earth should be added to a D.E. filter after cleaning or backwashing?: O A. 10 to 15 lbs. O B. 20 lbs. O C. 1 lbs. per 10 sq. ft. of filtration area of the filter 5. To lubricate, protect and make for easy reassembly and cleaning in the future a filter’s tank o-ring, each grid’s end piece and other screws and bolts that hold the filter together should be lubricated using: O A. Baby oil O B. Vaseline O C. Teflon based lubricant such as Magic Lube 6. When cleaning a D.E. filter always clean the grids off in a place that will not be seen by the customer or will not dirty the customer’s yard and also always clean up your work area and around the skimmer from any spilled D.E. powder because: O A. Leaving such mess in a customer’s yard is seen as careless and unprofessional O B. Leaving behind dirt and debris is not something we can charge extra for O C. Customers actually enjoy cleaning up our mess, so it is usually ok 7. A cartridge filter does not have a backwash feature to aid in keeping the filter clean: 135 O A. True O B. False 8. Cartridge filters do not use: O A. Chlorine in the water O B. Diatomaceous Earth to filter the water O C. Conditioner 9. A cartridge filter should be cleaned: O A. Every time the pool is vacuumed O B. Once every 6 months or as needed when the filter pressure increases O C. Whenever the mood strikes you 10. Sand filters should be backwashed about: O A. Once per week O B. Once per month O C. As the filter pressure increases O D. All of the above as needed Section 14: Different Types of Pool and Spa Surfaces: 1. Plaster is very delicate at start-up when a pool is first surfaced. You must NOT use a regular vacuum, only a brush-vac on a new plaster pool for at least: O A. 1 week O B. 5 weeks O C. 2 months 2. The chemicals for a brand new plaster start up must be added slowly, over__________ to prevent any staining to the new plaster. O A. 1 month O B. 1 year O C. 1 week 3. Most, but not all staining can be removed from a plaster surface by: O A. Super chlorinating 136 O B. Adding additional sodium bromine O C. Acid washing the surface 4. On grey and black plaster surfaces you will see calcium staining much more than on a white plaster surface. This calcium staining will create a white and grey mottling effect over the surface and can not be acid washed off. O A. True O B. False 5. A Pebble-Tech or Pebble-Sheen (smaller pebbles are used for Pebble-Sheen) surface must be brush-vaced at start-up: O A. True O B. False 6. The darkest shades of Pebble-Tech or Pebble-Sheen surfaces should be avoided when recommending the product as to avoid: O A. Noticeable calcium staining on the surface that can not be acid washed off O B. The warmer water temperatures caused by the darker color absorbing and retaining more heat from the sunlight O C. The textured surface 7. 3M Color Quartz, also called Diamondite Quartz and Quartzite is similar to plaster, however it is a combination mixed with a 3M quartz product giving a variety of vibrant colors, sometimes with blue or white crystal like specks mixed into it giving it more reflective properties in light, however it is very durable and can be start up without brush vacing just like: O A. Plaster O B. Gunnite O C. Pebble-Tech 8. 3M color quartz can be acid washed to remove surface staining: O A. True O B. False 9. Fiberglass is not made up of an alkaline material and does not give off additional Total Alkalinity from friction with the water. This makes a Fiberglass surfaced pool or spa VERY sensitive to: O A. Free Available Chlorine O B. Acid and pH changes O C. Conditioner, Cyanuric Acid levels 10. A more polished surface, such as fiberglass or an all tile surface is more reflective to sunlight and to the Sun’s harmful Ultra-Violet rays. These U.V. rays destroy chlorine. The U.V. rays 137 will reflect and bounce more in these types of pools affecting the chlorine more intensely. this means that: O A. The Free Available Chlorine level should be kept fairly strong, the conditioner level should always be at 100 ppm and a reliable chlorinator should be installed to help keep chlorine in the water O B. Less chlorine is needed O C. Less conditioner is needed 11. NEVER use a steel wire brush in a fiberglass, vinyl lined or all tile pool: O A. True O B. False 12. Always add as little acid as possible and be mindful of the pH balance when adding 3 inch tablets to a fiberglass or all tile pool because: O A. The chlorine level is too easily raised O B. These surfaces are VERY sensitive to acid and pH change O C. It will drop the conditioner out of circulation causing surface staining 13. When cleaning a vinyl lined pool always be VERY careful as not to: O A. Add too much sodium bromine O B. Over chlorinate O C. Tear or puncture the liner 14. ALWAYS use a steel wire brush to brush a vinyl lined or painted pool: O A. True O B. False 15. A painted pool can possibly be sensitive to acid and pH change, but not always, depending on the type of paint used and how glossy or reflective it is. Each painted surface can react to acid differently. O A. True O B. False Section 15: Types of Surface Staining: 138 1. Copper staining on the surface of the pool looks like: O A. Light to dark mottled and patchy turquoise blue stains on the surface O B. Green and orange spotting O C. Yellow powdery staining along the walls 2. When copper staining oxidizes it turns the existing blue copper stains on the surface: O A. Purple O B. Red O C. Grey to black 3. Iron staining on the pool’s surface is: O A. Orange-ish red to brown, often along the walls O B. A deep grey to black color, usually on the floor O C. White 4. Calcium is usually noticed along the water line or any spill ways and areas that get a lot of splash. Such calcium is caused by evaporation as when the water evaporates only the water itself, H2O, goes. The calcium, layer upon layer is left behind, day after day, minute after minute. Operating a pool durring non evaporation hours, early mornings or later evenings can help slow down the calcium deposit problem from evaporation as well as regularly coating the tile surface with an auto wax to help the water bead off more rapidly. Calcium also falls out onto the pool’s surface though we do not see it on white plaster pools. We can easily see it on colored pool surfaces. Other minerals and metals will stain onto calcium, using it like an anchor or a primer to adhere to more easily. Calcium also does not acid wash off as it is more dense than both the plaster surface and the grout in the tile and more damage will be done to the surface than to the calcium itself. What color is calcium when it falls out of circulation? O A. Red O B. Blue O C. White 5. Conditioner will stain the surface, equipment and any plastics in the pool. What color is conditioner staining?: O A. Light to dark blue found mostly on the floor O B. A light powdery purple ranging from a dusty coat to deep lines on the walls O C. White nodule like build ups usually along the tile surface 6. Sequestering and chelating (“key-late-ing”) treatments bond with any dissolved metal in the water and keep it from precipitating as a stain. These chemical compounds are also referred to as ____________________. Some are strong enough to pull metal deposits off the surface 139 and back into solution, thereby removing the stain.: O A. Stain and scale inhibitors/preventors O B. Metal suspenders O C. Metal removers O D. All of the above 7. Draining and acid washing a pool will: O A. Dissolve a fine layer of the plaster away, thereby removing much of the surface staining, but not all depending on how deep the staining is O B. Super chlorinate the plaster, bleaching it back to its original color O C. Pull the stains out of the plaster while leaving the plaster itself unharmed Service Technician Manual Test Answers: Section 3: Answers: 1. C, 2. A, 3. D, 4. C, 5. C, 6. A, 7. C, 8. D, 9. C, 10. E Section 4: Answers: 1. A, 2. C, 3. C, 4. B, 5. A, 6. A, 7. C, 8. A, 9. A, 10. D, 11. B, 12. A, 13. A, 14. B, 15. B, 16. B, 17. A, 18. B, 19. B, 20. C Section 5: Answers: 1. B, 2. F, 3. B, 4. A, 5. C, 6. B, 7. G, 8. B, 9. A, 10. B Section 6: Answers: 1. C, 2. B, 3. B, 4. A, 5. C, 6. D, 7. C, 8. C, 9. F, 10. B 140 Section 7: Answers: 1. C, 2. C, 3. D, 4. A, 5. A, 6. C, 7. B, 8. C, 9. A, 10. C Section 8: Answers: 1. C, 2. B, 3. A, 4. A, 5. D, 6. C, 7. B, 8. A, 9. C, 10. B Section 9: Answers: 1. A, 2. D, 3. D, 4. A, 5. B, 6. A, 7. E, 8. B, 9. C, 10. B, 11. D, 12. D, 13. D, 14. A, 15. A Section 10: Answers: 1. C, 2. A, 3. B, 4. B, 5. A, 6. B, 7. A, 8. A, 9. B, 10. A, 11. D, 12. A, 13. C, 14. A, 15. A, 16. E, 17. C, 18. A, 19. C, 20. C Section 12: Answers: 1. B, 2. A, 3. A, 4. C, 5. A, 6. C, 7. A, 8. C, 9. A, 10. B Section 13: Answers: 1. C, 2. B, 3. A, 4. C, 5. C, 6. A, 7. A, 8. B, 9. B, 10. D Section 14: Answers: 1. B, 2. A, 3. C, 4. A, 5. B, 6. A, 7. C, 8. A, 9. B, 10. A, 11. A, 12. C, 13. C, 14. B, 15. A Section 15: Answers: 1. A, 2. C, 3. A, 4. C, 5. B, 6. D, 7. A 27. Glossary of Swimming Pool and Spa Service Terms Acid Chemical compound characterized by sour taste and a pH less than 7 with the ability, when in solution, to react with a base to form a salt and to turn litmus red. Also, a substance that releases hydrogen ions (H+) in water. In pool water maintenance Muriatic Acid and Sodium Bisulfate (Dry Acid), also an acid, are used to lower pH and alkalinity. 141 Acid Demand A measure of the amount of acid required to reduce pH to a predetermined level. This can be accomplished using an acid titration procedure, referred to as an Acid Demand Test. Algae Tiny, hardy, plantlike organisms that will rapidly proliferate in water absent adequate sanitizer. Algae do not cause diseases but can harbor bacteria that do. Green-, black-, and mustard-colored algae are commonly seen in problem pools and spas. Here, besides being aesthetically displeasing, algae are a safety concern because they create slippery surfaces and can make it impossible to see a swimmer in trouble. Algaecide A chemical used to kill algae. Algaestat A chemical used to inhibit the growth of algae. Alkalinity See TOTAL ALKALINITY. Bacteria Potentially disease-causing, one-celled organisms requiring control by sanitizing agents. Balanced Water Water that is chemically stable, that is, neither corrosive nor scaling forming. The Langelier Saturation Index (LSI) for perfectly balanced water equals zero. Base Chemical compounds characterized by bitter taste and a pH greater than 7 with the ability, when in solution, to react with an acid to form a salt; also referred to as alkalis. Also, a substance that releases hydroxyl ions (OH-) in water. In pool water maintenance two bases are commonly used: Soda Ash (Sodium Carbonate), to increase pH, and Baking Soda (Sodium bicarbonate), to increase Total Alkalinity. Base Demand A measure of the amount of alkali material required to raise pH to a predetermined level. This can be accomplished by use of a base titration procedure, referred to as a Base Demand Test. Breakpoint Chlorination Dosing water with sufficient free chlorine to remove combined chorine (chloramines). Dosage is calculated as 10 times the combined chlorine value. Bromamines Byproducts formed when bromine reacts with contaminants in pool and spa water; unlike chloramines (combined chlorine), bromamines are effective sanitizers and do not need to be 142 eliminated. Bromine A compound containing the halogen bromine which is used to kill microorganisms in water. Calcium Hardness A measure of the calcium salts dissolved in water. Calcium hardness is the main component of scale. Chloramines See COMBINED CHLORINE. Chlorine A compound containing the halogen chlorine which is used to kill microorganisms in water. Chlorine in its natural state is a greenish-yellow gas. In swimming pool water chlorine serves as both an oxidizer and a sanitizer. Chlorine Demand Amount of chlorine required to eliminate all contaminants in the water. Chlorine Residual The amount of chlorine still available for sanitation and oxidation after the chlorine demand has been satisfied. Combined Chlorine Smelly, irritating reaction product of free chlorine with ammonia and other nitrogen-based contaminants in water; also known as chloramines. Conditioner Another term for cyanuric acid, which is used to slow down the degradation of chlorine by the UV component of sunlight. See STABILIZER. Corrosion An eating away of a material caused by chemical reactions. Water low in calcium hardness can be corrosive; it will dissolve metals, etch plaster, and pit concrete. Cyanuric Acid Chemical used to prevent the degradation of chlorine by ultraviolet (UV) light. Disinfect To kill all disease-causing organisms. Free Chlorine Chlorine available to sanitize and oxidize water; also known as free available chlorine. Total Chlorine The sum of free chlorine and combined chlorine. Halogen 143 An element found in Group VII of the Periodic Table, including chlorine, bromine, and iodine, characterized by the ability to disinfect water. Hardness (Hard Water) A property of water that contains ions which form scale. “Total hardness” is considered to be the sum of calcium and magnesium hardness. In water treatment, calcium and magnesium hardness values are usually expressed as calcium carbonate to facilitate treatment calculations. Hardness can be removed by ion exchange softening. Muriatic Acid Also known as hydrochloric acid; used to reduce pH and alkalinity. May be used to remove stain and scale. Oxidizer A compound (e.g., chlorine, bromine, potassium peroxymonopersulfate) used to “burn up” organic contaminants in water. See SHOCKING. pH A measure of the acidity /basicity of an aqueous solution. The pH scale runs from 0 to 14 with 7 being the neutral midpoint. A pH of less than 7 is on the acid side of the scale; a pH greater than 7 is on the basic (alkaline) side of the scale. Phosphates Phosphates are the microscopic organic food for algae. If there are too many phosphates in the water than algae can grow to be very healthy, hardy and resistant to chlorine. Potassium Potassium Peroxymonopersulfate is a powerful oxidizer and water clarifier. It is also sold as a non-chlorine “shock” treatment. ppm Abbreviation for parts per million. Sanitize To kill all living organisms, including algae, harmful bacteria, and other pathogens. Scale A buildup of crusty mineral deposits principally composed of calcium carbonate. Scaling water in piping will gradually reduce circulation, and will inhibit heat transfer. Shocking The periodic addition of an oxidizing chemical to rid pool and spa water of organic contaminants such as body oils, perspiration, personal care products, dust and dirt, etc. A non-chlorine shocking agent, such as potassium monopersulfate, cannot leave a sanitizing residual whereas the various chlorine compounds used for this purpose, such as calcium hypochlorite, can; however, use of a non-chlorine shock will allow swimmers to reenter the water sooner. See SUPERCHLORINATION. Soft Water Water lacking the ions which form scale. May be achieved by ion exchange softening. See HARDNESS. 144 Soda Ash Also known as sodium carbonate, used to raise pH levels. Sodium Bicarbonate Also known as Baking soda, used to raise total alkalinity levels. Sodium Bisulfate Chemical used to lower pH and total alkalinity; also known as dry acid. Sodium Sesquibicarbonate An even mix of soda ash and sodium bicarbonate, used to raise pH balance and total alkalinity levels simultaneously. Stabilizer Another term for cyanuric acid, which is used to slow down the degradation of chlorine by the UV component of sunlight. See CONDITIONER. Superchlorination Application of a large dosage of chlorine all at once to destroy built-up chloramines and scour the pool of oxidizable organic contaminants, including a green algae bloom. Typically the chlorine level would be raised to 30 ppm and then allowed to fall back gradually to below 5-10 ppm before swimming resumes. See SHOCKING. Total Alkalinity A measure of the acid-neutralizing capacity of water which indicates its buffering ability, i.e., resistance to changes in pH. Generally, the higher the total alkalinity, the greater the resistance to pH change. Total Dissolved Solids (TDS) The measure of all solids dissolved in a sample of water. Ultra Violet Rays (U.V.) The Sun’s harmful Ultra Violet rays can cause the degradation of sanitizers such as chlorine and sodium bromine and bounce within reflective pool surfaces such as fiberglass. 28. Final Notes from the Author You know, I have been servicing pools for over 13 years at the time that I had written and finished the 2nd edition of this guide and I still learn new things about this industry, service, chemistry and people every day. Don’t expect to learn everything in this guide in one reading. Many of these topics will take time and experience in the field to truly develop. 145 As long as this guide has been helpful and made your service experience easier and more efficient than it has served its purpose. There are many technicians out there who will disagree with some or even many of the things in this guide, however it is not meant to tell you what to do, so much as it is to guide you along in your development as a high quality and knowledgeable swimming pool and spa service technician. Much of what was written in this manual was based on actual experience from the field and what works where it counts, not in a laboratory or perfect world swimming pool conditions. In the end use your better judgment and common sense. Do not forget that there are always many resources available to you. Much of what I have learned has been from others. I am happy to have the opportunity to share what I have learned with you and I encourage you to share the things that you learn with each others as well. Thank you, sincerely for reading this guide. I am not a writer, but I do hope that this guide has been easy to follow. If you have any comments, concerns, corrections or ideas you can email me at: will_scott1@yahoo.com Thank you again for reading it. Sincerely, Jansen T Falvai This free copy of Swimming Pool & Spa Service Training Guide has been provided to you by: 146 Sales Manager

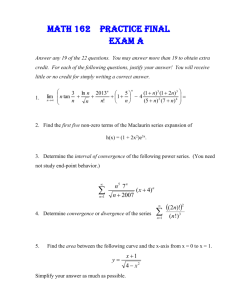
![[1] - Boswellsgmt](http://s3.studylib.net/store/data/006603407_1-fadfbce8d94050a9fb3c38a07d86e8ee-300x300.png)

