Memorandum of Understanding/Trial Participation Agreement
advertisement

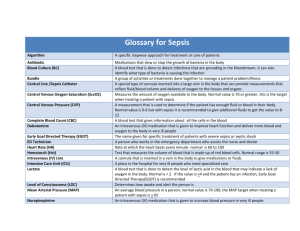
Memorandum of Understanding/Trial Participation Agreement Assessment of Improvement Methodology in Sepsis This agreement is effective as of this _____day of _________, 2011, by and among ______________ Hospital (“Hospital”), _______________, Site Principal Investigator (“Site PI”) and the AIMS Study Investigators (“AIMS”) for the purpose of Hospital and Site PI’s participation in the “Assessment of Improvement Methodology in Sepsis” (AIMS) Study. Project Description: Sepsis is a leading cause of death in hospitalized patients and costs approximately $17 billion to the U.S. health system annually. Using the evidence-based elements of the Surviving Sepsis Campaign bundles has been shown to be a cost-effective approach to significantly decreased mortality of patients with severe sepsis or septic shock. The AIMS Study is an innovative timed, cluster randomized controlled trial of two quality improvement strategies for implementing the Surviving Sepsis Campaign bundles. Upon enrollment, hospitals will be randomized to either a multi-faceted collaborative quality improvement approach or a program of intensive continuing medical education (CME). After 18 months, hospitals initially randomized to intensive CME will receive the multifaceted collaborative quality improvement intervention. All sites will participate in collaborative quality improvement; the timing of the start of the collaborative will be randomized. Participation in this initiative will provide your hospital with important evidence on selecting improvement efforts for lowering mortality, complications, and costs associated with severe sepsis and septic shock and as a model for other conditions. In addition, participation provides an opportunity to assume a leading role in a national study in the field of quality improvement. AIMS Investigators agree to provide: A multi-faceted, collaborative quality improvement intervention led by investigators from the Surviving Sepsis Campaign which will include: o Monthly collaborative conference calls with study experts o Reports on hospital adherence to evidence-based bundle elements o Updated scientifically rigorous evidence-based sepsis bundles o Regional launch meeting with study investigators o Updated educational materials and website o Study list-serve to communicate and share ideas with other study participants o 24/7 availability of sepsis collaborative team (MD, RN, and database support) o Access to updated Surviving Sepsis Campaign database o Data on your hospital’s compliance with sepsis bundles to inform your efforts toward improving care for patients with severe sepsis and septic shock while in the Collaborative o Monthly CME opportunity to hospitals randomized to begin the study with the CME intervention Laptop computer with pre-installed database for data entry Maintenance of HIPAA compliant, de-identified data on a secure server Hospitals and Site PI’s agree to: Be randomized as to timing of participation in a multi-faceted collaborative quality improvement intervention to improve care for patients with sepsis Commit to improve the care of patients with severe sepsis and septic shock within the institution Identify an interdisciplinary team of Emergency Department (ED) and Intensive Care Unit (ICU) physicians and nurses to: o serve as local champions for sepsis quality improvement o implement collaborative interventions in their hospital o attend a regional launch meeting and monthly conference calls Enter complete and accurate data on evidence-based sepsis bundle elements on all patients admitted to the ICU with severe sepsis or septic shock during the study period (up to 4-5 years) Provide adequate staff time to enter the data described above Enter the above data for the duration of the study period Authorized Hospital Representative: Name: _ _ Hospital: ___ Title: Date: _________ Site Principal Investigator: Name: _ _ Hospital: Title: Date: ______________ AIMS Study Investigator: Name: _ Hospital: Title: Date: ______________ _