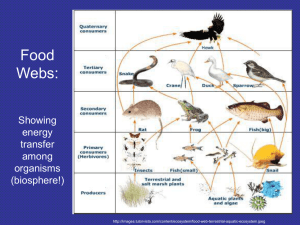
Contents - Micronutrient Initiative
advertisement

PNG National Micronutrient Survey 2005 Papua New Guinea National Micronutrient Survey 2005 1 PNG National Micronutrient Survey 2005 Contents Map of the four regions of PNG ....................................................................................................... 5 Investigators and Collaborators ...................................................................................................... 6 1. Introduction ................................................................................................................................... 7 1.1 Background ............................................................................................................................... 7 1.2 Justification for the survey and the intended and potential uses of the survey data ................... 8 1.3 Objectives ................................................................................................................................. 8 2. Planning and preparation ............................................................................................................. 9 2.1 Study coordination .................................................................................................................... 9 2.2 Components of the Survey ...................................................................................................... 12 2.3 Target populations .................................................................................................................. 12 2.4 Community Mobilization .......................................................................................................... 13 2.5 Survey Teams ......................................................................................................................... 13 2.6 Training ................................................................................................................................... 14 3. Sampling...................................................................................................................................... 14 3.1 Stratification ............................................................................................................................ 14 3.2 Sample size determination ...................................................................................................... 15 3.3 First stage sampling ................................................................................................................ 17 3.4 Second stage of sampling ....................................................................................................... 17 4. Data collection ............................................................................................................................ 17 4.1 Inclusion and Exclusion procedures ........................................................................................ 17 4.2 Enrollment procedures and consent ........................................................................................ 18 4.3 Data collection at the household ............................................................................................. 19 4.4 Validation study ....................................................................................................................... 20 5. Survey Instruments and outcomes............................................................................................ 20 5.1 General Questionnaires .......................................................................................................... 20 5.2 Modified Fortification Rapid Assessment Tool (FRAT) ............................................................ 21 5.3 Anthropometry ........................................................................................................................ 21 5.4 Anemia .................................................................................................................................... 24 5.5 Iron status and Iron deficiency anemia .................................................................................... 24 5.6 Vitamin A status ...................................................................................................................... 25 5.7 Iodine status............................................................................................................................ 25 2 PNG National Micronutrient Survey 2005 5.8 Malaria .................................................................................................................................... 26 5.9 Hookworm ............................................................................................................................... 26 6. Specimen Processing ................................................................................................................. 26 6.1 Hemoglobin cuvettes ............................................................................................................... 27 6.2 DBS processing ...................................................................................................................... 27 6.3 Malaria slides .......................................................................................................................... 27 6.4 Stool........................................................................................................................................ 27 6.5 Urine ....................................................................................................................................... 27 6.6 Salt.......................................................................................................................................... 27 7. Lab Procedures ........................................................................................................................... 28 7.1 TfR and CRP ........................................................................................................................... 28 7.2 Retinol..................................................................................................................................... 28 7.3 Malaria .................................................................................................................................... 28 7.4 Hookworm ............................................................................................................................... 28 7.5 Urinary iodine .......................................................................................................................... 28 7.8 Salt.......................................................................................................................................... 28 7. Data entry and analysis plan ...................................................................................................... 29 8. Dissemination of study results .................................................................................................. 29 9. Time frame................................................................................................................................... 30 Appendix 1: List of publications by principal investigators ........................................................ 31 Appendix 2: Sample Size Breakdown ........................................................................................... 36 Appendix 3: Supplemental information for protection of human research participants ........... 37 Appendix 4: Time Frame ................................................................................................................ 39 Appendix 5 ...................................................................................................................................... 42 3 PNG National Micronutrient Survey 2005 Acronyms AGP 1-acid glycoprotein CRP C - reactive protein DBS Dried Blood Spot DOH Department of Health MDG Millennium development goals MTSP Mid term strategic plans PNG Papua New Guinea PNGNMS Papua New Guinea Nutrition and Micronutrient Survey PSU Primary sampling Unit TfR Transferring Receptor UPNG University of Papua New Guinea 4 PNG National Micronutrient Survey 2005 Map of the four regions of PNG Momase Region Islands Region Highlands Region Southern Region Momase Region Islands Region Highlands Region Southern Region 5 PNG National Micronutrient Survey 2005 Investigators and Collaborators Support to the Department of Health (DOH) for this survey will be provided by several investigating and collaborating agencies. These include (in alphabetical order): CRAFT Technologies Mahidol University, Thailand SEAMEO TROPMED Regional Center for Community Nutrition (RCCN) The University of Papua New Guinea (UPNG) UNICEF Papua New Guinea (UNICEF PNG) U.S. Centers for Disease Control and Prevention (CDC) The core members of the survey coordinating committee are as follows: Florence Addo (UNICEF PNG) - Survey Coordinator Dr Gilbert Hiawayler (DOH) – Coordinator Data analysis and collection Dr Victor Temple (UPNG) – Laboratory coordinator Enoch Posani (DOH) - Chair Wila Saweri (DOH) – Survey Advisor The core survey coordinating committee will be supported by the following members of the International Micronutrient Malnutrition Prevention and Control Program (IMMPaCt) at the USA Centers of Disease Control and Mahidol University, Thailand. Katie Tripp Dr Bradley Woodruff Tracy Dearth-Wesley Bridgette Bowden Dr Rosemary Schleicher Dr Pattanee Winichagoon Please see appendix 1 for a list of the relevant publications by the principal investigators 6 PNG National Micronutrient Survey 2005 1. Introduction At the beginning of 2004, the Government of PNG expressed interest in attending a training course on conducting national micronutrient surveys, with the expectation of conducting their own national survey in 2004-2005. The course was led by the CDC in Bangkok Thailand, in March 2004. A team of 4 people from PNG attended the training. Based on the initial plans outlined by the team, the International Micronutrient Malnutrition Prevention and Control (IMMPaCt) program at the CDC provided funding and technical assistance for the micronutrient survey, under the terms of a cooperative agreement between the CDC and UNICEF, to work towards the elimination of micronutrient deficiencies. UNICEF PNG also agreed to contribute funding and personnel to assist with planning, implementation and programmatic follow up to the survey. A meeting to plan the survey was convened in July 2004 in Port Moresby, Papua New Guinea. Participants included officials from the DOH, UPNG, CDC, Mahidol University, Thailand and UNICEF PNG. Members of the team included Enoch Posani DOH; Gilbert Hiawalyer, Director of Monitoring and Research Branch, DOH; Wila Saweri, Technical Advisor Nutrition and Family Health Services, DOH; Victor Temple, Professor, School of Medicine and Health Sciences; Pattannee Winichagoon, Associate Professor, Institute of Nutrition, Mahidol University; Katie Tripp, Micronutrient Specialist, IMMPaCt, CDC; Bradley Woodruff, Medical Epidemiologist, IMMPaCt, CDC; and Dan Sadler, CDC. The final plan for the Papua New Guinea National Micronutrient Survey (PNGNMS) was developed through wide consultations with micronutrient stakeholders and experts both in PNG and at CDC. 1.1 Background Malnutrition in all its forms affects socio–economic development in PNG. Nutrition plays a critical role in the survival, growth, health and development of children, who do not reach their full potential if malnourished in their early years. In addition, a supply of adequate micronutrients is crucial for optimal health. The development goals as contained in Medium Term Strategic Plan (MTSP), the Millennium Development Goals (MDGs), and other plans cannot be achieved if malnutrition remains a public health issue. PNG is a country in transition. While a large proportion of the country is still affected by undernutrition, people increasingly succumb to lifestyle related diseases like diabetes, heart ailments and smoking related diseases. Since Independence in 1975, large mining and oil exploration projects have provided a substantial income for landowners and the government. In addition, cash crops, like oil-palm, coffee and vanilla, have provided an income for the rural population. Adaptation to modern lifestyle of Papua New Guineans affects their food habits and food choices. Although this transition is not uniform across the country, the change is greatest in areas, where people have access to ready cash 7 PNG National Micronutrient Survey 2005 As a result, eating habits have changed from the traditionally grown subsistence diet to a diet containing an increasing proportion of commercial foods. For example, rice is no longer the staple food of only urban dwellers and is increasingly consumed by farmers who cultivate rice as a cash crop or purchase it in the market. 1.2 Justification for the survey and the intended and potential uses of the survey data As no national nutrition survey has been undertaken since 1982-83, the impact of these dietary changes on nutritional status is not known. Furthermore, as the last survey was only conducted on children under 5 years of age and no measures of micronutrient deficiencies were taken, there are no national data on the micronutrient status of the population of PNG. Nonetheless, data on local and selected populations show that micronutrient malnutrition may be common in at least some populations in PNG. In order to effectively reduce malnutrition, including micronutrient deficiencies, there is need to know the magnitude and the determinants of nutritional problems. In 1995 PNG amended the Pure Food Standards and all salt must be iodized however, the coverage of fortified salt has never been determined nationally. Some small surveys show a wide variation in estimates of coverage in different locations. Moreover, in 2002 supplementation with high doses of vitamin A was introduced for children less than 5 years of age every 6 months, but the program's coverage or effectiveness is unknown. Thus, a national survey is needed to: Generate data for evidenced-based advocacy, social marketing and resource mobilization for micronutrient interventions including food fortification and vitamin and mineral supplementation; - Evaluate the coverage of salt iodation and vitamin A supplementation interventions; and - Provide baseline data for planning, programming, monitoring and evaluating micronutrient interventions. 1.3 Objectives The overall goal of the PNGNMS is to assess the overall micronutrient status of various population groups and to evaluate current interventions to improve micronutrient status in PNG; to enable the DOH and its to more effectively manage existing interventions and to adequately plan, implement and monitor new prevention programs and evaluate their impact. The specific objectives are to obtain regionally (Southern, Highlands, Momase and Islands) representative estimates of: 8 PNG National Micronutrient Survey 2005 the household coverage of adequately iodized salt the urinary iodine levels among non-pregnant women of child bearing age (15-49 years of age) the prevalence of anemia and iron deficiency in children 6-59 months of age and non-pregnant women of child bearing age the prevalence of vitamin A deficiency in children 6-59 months of age and nonpregnant women of child bearing age the prevalence of stunting and wasting in children 6-59 months of age the prevalence of underweight and overweight in non-pregnant women of child bearing age and men over 18 years of age. the prevalence of anemia in men 18 years of age and above the contribution of malaria and hookworm to anemia. to obtain nationally representative data on the use and consumption levels of centrally-processed staple foods, in order to determine their suitability as vehicles for fortification 2. Planning and preparation 2.1 Study coordination The PNG survey coordinating committee has overall responsibility for the quality of data collection and analysis and for timely and appropriate reporting and advocacy. Tasks of this committee include: On-going planning and implementation of the PNGNMS Ensuring adequate financial, human and organizational resources for the PNGNMS) 9 PNG National Micronutrient Survey 2005 Ensuring timely data analysis, report writing and dissemination Organizing a high level meeting to advocate for action based on the results of the PNGNMS The coordinating committee will hold the meetings to: Review and finalize the draft study protocol, to discuss and approve a work plan and ensure adequate resources are available to implement this plan Discuss the draft report presenting the results of the PNGNMS, plan the meeting to disseminate the final results and plan additional activities to implement preventive interventions or further studies The Coordinating Committee will also hold regular meetings to discuss the progress of the PNGNMS and any problems that need to be resolved. Members of the committee include representatives of the DOH, UPNG and UNICEF PNG. Table 1. Allocation of Roles and Responsibilities by Institution. Task Responsible Finalizing study protocol DOH, UNICEF (CDC available to assist) Consultant: Hiring UNICEF Supervising DOH, UNICEF Procuring supplies UNICEF, UPNG Laboratory: Collecting and handling of specimens UPNG Analysis of specimens UPNG, CRAFT, SEAMEO, CDC Logistics: 10 PNG National Micronutrient Survey 2005 Developing a work plan Hiring and training interviewers DOH, UNICEF Transport Informing/involving district Payment of staff Data management: Data management plan Data entry DOH, NSO, CDC Data analysis Obtaining research and ethical clearance DOH Finances: Arrange funding from UNICEF NY and reporting to donor Financial management Accounting and financial reporting UNICEF PNG DOH, UNICEF DOH, UNICEF Report writing and dissemination of results: Write the survey report Print the survey report Organize dissemination and advocacy events DOH/UNICEF PNG/ UPNG (CDC available for assistance) A survey coordinator will be hired and will be responsible for the day-to-day planning and implementation of the PNGNMS. The survey coordinator will report to the National Micronutrient Study Coordinating Committee. UNICEF PNG will be responsible for hiring the Survey coordinator. Qualifications of the successful applicant should include: An MSc or higher degree in a medical, nutrition or bio-chemical field (a PhD would be an added advantage) Expertise in and experience with planning and conducting field-based nutrition or health surveys Experience with coordinating multi-sectoral teams and working to a detailed timeline 11 PNG National Micronutrient Survey 2005 Experience with obtaining government approval for activities and with ordering supplies and obtaining customs clearance Experience with data analysis, especially analyses using SPSS or EPI-info Experience with data interpretation and report writing Availability full time for a period of 1 year Self motivated and able to work independently and as part of a team Besides technical assistance for the specific activities of the PNGNMS (e.g. sampling, data analysis, and laboratory analysis), CDC will make a person available who will work closely with the survey coordinator at critical points in the survey, such as finalizing the protocol, developing the questionnaires, training the survey workers, supervising field data collection, data analysis and interpretation, and writing the report). 2.2 Components of the Survey The PNGNMS will be composed of integrated components that will be used to assess the nutrition micronutrient status of the target groups. The various methods to be employed include anthropometric measurements, hematological and biochemical assessments, salt sample analysis and interview questions. A modified Fortification Rapid Assessment Tool (FRAT) will also be utilized to gather further information on potential vehicles for food fortification. 2.3 Target populations The target populations to assess anemia, iron, and vitamin A status are children 6 to 59 months of age and non-pregnant women of child-bearing age as these groups are the most vulnerable to deficiencies of iron and vitamin A. Men are at substantially lower risk of many nutritional deficiencies and are therefore not specifically targeted in most micronutrient assessments. However, hemoglobin status will also be assessed in adult men 18 years of age and older in the PNGNMS in order to ascertain whether anemia is due to iron deficiency and/or other factors. Nonetheless, men may be included in such surveys because they are much less susceptible than women and children to iron deficiency, but equally susceptible to other causes of anemia. Therefore, survey findings indicating that anemia is common among women and children but rare among men provide evidence that iron deficiency is a predominant cause of anemia. 12 PNG National Micronutrient Survey 2005 Anthropometric measurements will be taken for all eligible children (6-59 months), women 15-49 years and men (18 years and over). 2.4 Community Mobilization Before commencing data collection, officers from the DOH and UNICEF, in coordination with the provincial government offices, will organize and implement a community mobilization campaign. These organizations will produce information papers and radio spots and send this information to all communities selected to take part in the survey so that people will know well ahead of time when the data collection for the survey will occur and appreciate its importance. 2.5 Survey Teams Six field teams will collect the data for the PNGNMS. Each team will consist of one team leader, one interviewer, one lab technician, and one anthropometrist. One regional level supervisor will also be recruited to help with organization and transport of supplies and laboratory specimens. Some survey workers will come from the various organizations participating in the PNGNMS, such CDC, DOH and UPNG. Please see table 2 for a description of team roles and responsibilities. The community mobilization system will be arranged to ensure that the chief of each selected village will have been informed and will notify selected households when the survey teams will arrive. Where possible additional advance notice will be sent to each selected village just before the arrival of the survey team. Table 2. Roles and responsibilities Role Responsibilities Team Leader Identify selected households, Maintain records and cluster control sheets, Check labels and completed data collection forms Plan revisits. Laboratory technician Perform finger sticks and phlebotomy Manage laboratory specimens during data collection Collect urine and stool specimens Anthropometrist Measure height and weight of children and women 13 PNG National Micronutrient Survey 2005 Interviewer Interview respondents Assist in weighing and measuring women and children Collect household salt specimen 2.6 Training All team members will receive 8 days of instruction regarding the overall survey objectives and procedures. Some components of the training include: Interviewers responsible for administering the survey questions will participate in roleplaying interviews to insure consistency. Interviewers will also complete a standardization exercise for anthropometric measurements so that they can assist the anthropometrists. Interviewers will also be trained on how to collect, label and store the specimen of household salt. Laboratory technicians will be trained by CDC and UPNG lab personnel on procedures for performing a finger stick, the preparation of the filter paper dried blood spot, the use of the HemoCue™ photometer for hemoglobin measurement, the preparation of a thick blood smear for malaria, and the collection and field processing of urine and stool specimens. Anthropometrists will be trained to measure weight and height or length on children and adults. This training will include practice on both adults and children and a standardization exercise measuring height or length of children. There will also be two days pilot testing of all survey procedures before data collection begins to ensure that all survey procedures are well understood. 3. Sampling 3.1 Stratification A two-stage cluster sampling design will be used with stratification to generate national estimates for the major nutrition outcomes as well as region-specific estimates for the four main regions (Southern, Highlands, Momase and Islands). The stratification by these four regions will be done for the following reasons: The diversity of the landscape, and agriculture and cultural practices may result in wide 14 PNG National Micronutrient Survey 2005 differences in the nutrition outcomes among the regions. Programs may need to be introduced or targeted regionally and region-specific estimates could help identify those regions in greatest need of interventions. The credibility of the survey findings at a regional level will be enhanced. The 2000 census enumerated a total population of 5.2 million persons with 1,041,820 (20%) residing in the Southern region, 1,973,996 (38%)in the Highlands region, 1,433,432 (28%) in the Momase region and 741,538 (14%) in the Islands region. However, the PNGNMS will select the same sample size for each region. Because of the resulting differences in sampling fraction for each region, population data from the 2000 census will be used to weight the calculation of nationwide estimates of the nutrition outcomes. 3.2 Sample size determination The sample size for the PNGNMS was determined using standard statistical procedures. The anticipated prevalence, desired precision, and assumed design effect for each outcome in each target group were determined based on the results from previous surveys and studies related to the outcomes of interest. Sample sizes for each outcome in each target group were calculated using the standard formula: N = 1.962 x pq x DEFF d2 Where: N = Minimum sample size needed 1.96 = the z value to obtain a 95% confidence interval p = the assumed prevalence of the nutrition outcome of interest in a target group q = 1-p d = the desired precision expressed as a half-confidence interval DEFF = the design effect to account for the loss of statistical precision from cluster sampling For many nutrition outcomes, conservative assumptions were made to intentionally overestimate the necessary sample size. For example, because the sample size is maximum if the prevalence is 50%, if the prevalence of a specific outcome was thought to possibly approach 50%, the sample size calculation assumed 50% as the prevalence. Similarly, design effects were overestimated to ensure adequate sample sizes. For example, the calculation of a sample size for anemia in children 6-59 months of age was based on an estimated anemia prevalence of 50%, a precision of +/-10 percentage 15 PNG National Micronutrient Survey 2005 points, and a design effect of 2. Using the standard formula above, 193 children would be needed per region. The nationwide sample would require 4 times as many children 659 months of age because there are 4 strata, thus resulting in a total of 768 children. But, of course, a certain proportion of selected households will be unavailable or refuse participation (household non-response) and a certain proportion of children in consenting households will be absent or their mothers will refuse consent for a fingerstick (individual non-response). Taking into account an estimated individual non-response of 20%, a household non response of 10%, and the proportion of children 6-59 months of age per household in PNG (0.7 children per household), the required number of households that need to be selected to obtain fingerstick blood on 768 children is 1, 524 (768 / 0.80 / 0.90 / 0.7). For most of the outcomes and target groups of interest, there are very few data on which to base the assumptions necessary to calculate sample size. As described above, a prevalence of 50% was selected for such indicators to provide the largest sample size for the given target population. (See appendix 2 for a detailed description of the assumptions used to generate the sample sizes for all major outcomes) For a given target group, sample sizes were calculated separately for each nutrition outcome measured in that group, and the maximum size was taken for that target group. We decided that the number of households for the entire survey should be 1600 households, as this will provide at least the desired precision for most of the nutrition outcomes of interest. Table 3 shows the number of target individuals who will be included in the sample of 1600 HHS. Table 3. Estimated Number of participants by target group in strata sample of 400 households and total sample of 1600 households Target group Indicators Number of expected samples from participants per Strata Total number of participants nationally (4 strata in total) 1600 households Children 6-59 months Laboratory 202 806 Anthropometry 227 907 Nonpregnant women 15 49 years* Laboratory 197 789 222 888 Adult men 18 years and above* Laboratory 203 810 243 972 Anthropometry Anthropometry * On ½ of all house holds ** These figures take into account the expected presence of participants at the household and the household individual response rate. 16 PNG National Micronutrient Survey 2005 At every household visited anemia, iron deficiency, malarial load, wasting and stunting will be measured in each child in the household. In every second household anemia, iron deficiency, BMI, urinary iodine, malarial load and hookworm will be measured in all nonpregnant women of child-bearing age, and anemia will be measured in all men above 18 years of age, giving a total of 100 sampling units nationally. 3.3 First stage sampling The National Statistical Office provided a list of all census units in PNG in an Excel spreadsheet. For the first stage of sampling, the sampling unit, and therefore the primary sampling unit for the PNGNMS, was a census unit. For each of the four regions, 25 primary sampling units were selected probability proportional to size. These randomly selected primary sampling units are located in all 20 provinces and in 75 of the 87 districts in PNG: 16 districts in Southern region, 24 in the Highlands regions, 23 in the Momase region and 12 in the islands region. Although all the provinces will be included in the survey, it is important to note that the precision around the estimates of the prevalence of all outcomes will not be sufficient to interpret the results of the survey by province. 3.4 Second stage of sampling Before data collection begins the survey coordinator will work with officers from the National Statistical Office to obtain accurate and complete lists of the households in each selected primary sampling unit. In large primary sampling units (great than 250 house holds) where it is not possible to make a list of all households, a sub-section of the primary sampling unit will be chosen at random probability proportional to size. A list of all the households in this subsection will then be created. In both smaller units with a complete list of all households and larger units where the list of households includes only a selected sub-section, simple or systematic sampling will be used to select households. In order to select a sample of 1600 households in total, 16 households will be selected from each of the 100 selected primary sampling units. Although the basic sampling unit is the household, the unit of analysis for all the nutrition variables is the individual child, woman or man in the household. In each selected household, all eligible persons in the identified target groups will be offered participation in the survey. For the PNGNMS, a household is defined as a group of people who share a common cooking pot. 4. Data collection 4.1 Inclusion and Exclusion procedures Of the 16 randomly selected households visited per sampling unit, households will be eligible for participation in the nutrition survey providing that the following criteria apply; 1) any one of the defined target groups are represented in the household, i.e. a child 617 PNG National Micronutrient Survey 2005 59 months of age, and/or a woman of child-bearing age 15-49 years of age, and/or a man 18 years and older, 2) the eligible person lives in the selected household at the time of data collection, and 3) consent is given by the participant or a responsible adult for survey participation. 4.2 Enrollment procedures and consent Upon arrival at the first selected household the survey team supervisor will ask whether any children 6-59 months live in the household. If there are no eligible children this will be recorded on the summary form for the HHs in the sampling unit and the team will go on to the second house. If there are eligible children at the first house, the team supervisor will then explain the purpose of the survey, and the methods and procedures to the head of the household and ask for consent to proceed with data collection in this household. At the second house, the survey team supervisor will ask whether any children 6-59 months, and/or women 15-49 years old, and/or men over 18 years old live in the household. If there are no eligible people, this will be recorded on the summary form for the HHs in the sampling unit and the team will go on to the third house. If there are eligible subjects in the second house, the team supervisor will then explain the purpose of the survey, and the methods and procedures to the head of the household and ask for consent to proceed with data collection in this household. For the third house (and every other next selected household following) visited, the procedure will be the same as for the first house. And for the fourth house (and every other next selected household following), the procedure will be as for the second household. All household members who fit the criteria for a target group will be included in the survey if verbal consent is received. Please see appendix 3 for more information on the consent process. At the start of the survey team's visit to selected household, household members or others in the community will be asked, where feasible, to fetch absent household members who are eligible for survey inclusion. When any of the selected eligible occupants of a house are not at home, up to three recall visits if possible may be made for another time, over subsequent days. Selected houses which are either vacant or in which all eligible occupants are away, will not be replaced. Survey teams will record for each selected household in the survey sample the following information, 1) whether the house was unoccupied or occupied, 2) whether at least one member of the target group was present (required target groups will differ for every other house) in the household, 3) how many eligible subjects from each group lived in the household, 4) whether household consent was given, 5) whether consent was given for 18 PNG National Micronutrient Survey 2005 each of the individuals selected for the survey in each house, 6) whether data collection was completed for each of these individuals, and, 7) if data were not collected from any of the selected subjects, why. These data will allow calculation of household weights, response rates and the determination of reasons for non-response. 4.3 Data collection at the household On arrival at the household the team leader will ask for the head of the household who is currently living in that property. All children 6-59 months identified in each of the selected households will qualify for participation in the survey, whether or not their mother is also taking part in the survey. The child’s mother or caretaker will be asked to answer the questionnaires on their behalf. All non-pregnant women 15-49 years in every second selected household will be included in the survey and every woman in these households will be asked to participate in the survey. All men aged 18 years and over in every other selected household will also be asked to participate. Once eligible household members have been identified, the interviewer for the survey team will start with the care taker of each eligible child. Then all eligible women and men will be interviewed. The interviewer will request that a 15 gramms sample of salt be taken from the house. The interviewer will provide iodized salt to replace the salt collected. After the interviews have been completed, children, women and men will be weighed and their height/length measured by the anthropometrist. These measurements will be recorded on the data collection forms. When the measurements are complete the women in the household will be asked to provide a urine specimen to assess urinary iodine. The laboratory technician will provide women with a screw top urine cup and specific directions regarding the urine collection process. If they are unable to provide a sample at that time they will be asked to drink some water, so that they might be able to provide a sample by the end of the blood collection. If there are any eligible men in the household the trained laboratory team member will collect capillary blood using a HemoCue Lancet. The first two drops will be wiped away and the third drop will be drawn into a HemoCue cuvette to evaluate hemoglobin. After all eligible men have been tested each eligible woman will have her finger pricked. The first four drops of blood will be dropped directly on to filter paper to create four dried blood spots, which will later be assessed for TfR, CRP and serum retinol. The fifth drop of blood will be drawn into a Hemocue cuvette for evaluation of hemoglobin by the HemoCue Hemoglobin System (HemoCue AB, Angelholm, Sweden). A final drop of blood will be used to create a thick smear for malaria assessment. After all eligible men and women have had their blood drawn all children included in the survey will also have capillary blood collected in exactly the same way as described 19 PNG National Micronutrient Survey 2005 above. For all survey participants having their hemoglobin measured the HemoCue cuvette will immediately be placed in the HemoCue and the measurement will be read off the instrument and recorded on the data collection form. All people participating in the survey will be told their hb value. Anyone with a hemoglbin concentration below the World Health Organization cutoffs (see section 5.4) will receive a referral to the nearest clinic. Stool will be collected from all eligible children between the ages of 24-59 months. Caretakers will be provided with cups with screw tops lids to collect the stool. If possible stool will be collected during the visit. If that is not possible then caretakers will be left with the cups and the survey team will re-visit the household once more before leaving that PSU, in order to collect the specimen. Once the interviews, anthropometry and biological specimen collection are complete the team will leave the house and move on to the next household on their list, where they will complete the same procedure as the one described above. Before leaving the household the team will ensure that all waste has been put in bio-hazard bags and lancets disposed of in the sharps collector. The team supervisor will also check the data collection form to ensure complete and accurate data recording. 4.4 Validation study In a sub sample of house holds in the 6 primary sampling units surrounding Port Moresby (NCD) children (24-59 months), women (18-59 years) and men (18 years and above) enrolled in the survey may be asked to provide a venous blood sample in addition to the samples described above. The survey staff may also volunteer for this study. The venous blood collected will be used to validate the DBS TfR, CRP and retinol collected from the rest of the survey. (See appendix 5 for details of the validation study). 5. Survey Instruments and outcomes 5.1 General Questionnaires The questionnaire portion of the data collection form will include questions on anemia, vitamin A and iodized salt. The questionnaire will be translated into Pidgin. In areas where pidgin is not widely understood the questionnaire will be translated verbally by a local person who can speak pidgin and the local language. Field workers administering the questionnaire will be hired from the areas where the survey will be conducted to provide on site translation of the questionnaire questions. Due to language barriers the questionnaire will be very brief and only aim to collect fact-based information. For example, interview questions may inquire about demographic questions (women’s age, reproductive status and history) and socioeconomic status questions (educational level, 20 PNG National Micronutrient Survey 2005 household socio-cultural background). Similarly the child questionnaire will include additional parameters on sex, age, breastfeeding status and complementary feeding. 5.2 Modified Fortification Rapid Assessment Tool (FRAT) The Fortification Rapid Assessment Tool (FRAT) method created by PATH Canada will be adapted for use in the survey. A national sample of each participant group (children, women and men) will be asked questions from the FRAT, specifically information on consumption with estimates of quantities of the already identified centrally processed foods to fortify (sugar and oil with vitamin A, rice, wheat flour and complementary foods with iron, folic acid and other nutrients). FRAT information will be gathered on a national sample of women men and children. In each sampling unit two women and two men and two primary caregivers of children included in the survey will be asked the FRAT questions. To ensure that FRAT information is gathered from 6 different households per cluster, women will be interviewed in the first two eligible houses visited, children’s care takers in the next two and men in the two houses after that. 5.3 Anthropometry Height and weight measurements will be taken for all non-pregnant women of child bearing age and all children 6-59 months of age included in the survey. Children’s ages will be determined from a health card or other documentation, if available. If no such documentation is available, a local calendar will be used to determine age to the nearest month. Anthropometric indicators of length/height-for-age, weight-for-age and weightfor-length/height will be determined for children (see box 1) and weight and height will be used to determine body mass index (BMI) in women (see box 2). a) Length/height For children less than 24 months old, recumbent length will be measured to the nearest 0.1 cm using a height board either manufactured or patterned on height boards made by Shorr Productions (Olney, Maryland, USA). The same height board will be used to measure standing height to the nearest 0.l cm for children greater than or equal to 24 months of age and for adult women. All subjects will be measured without shoes or hair accessories which add artificial height. b) Body weight UNICEF Seca Uniscales will be used to measure body weight of women of childbearing age 15-49 years and children 6-59 months. The weight of the children will be measured 21 PNG National Micronutrient Survey 2005 by taring the scale after the mother's weight is recorded, than handing the child to the mother BOX 1. ANTHROPOMETRIC INDICES Reference: Pediatric anthropometric data presented in this report were interpreted using the international growth reference (NCHS/CDC/WHO reference). This reference is based on growth curves for children in the United States and studies have demonstrated that healthy, well-nourished children from most countries exhibit a pattern of growth that is similar to that of the reference. Z-scores: The anthropometric indices used for evaluating the nutritional status of children include height-for-age, weight-for-age, and weight-for-height. These indices are interpreted using classifications based on Z-scores (standard deviation units from the reference median). The World Health Organization (WHO) recommends that a Z-score cut-off point of <-2 be used to classify low height-for-age, low weight-for-age, and low weight-for-height for estimating the prevalence of malnutrition. The reference Z-score distribution for each index has a mean of 0.0 and a standard deviation of 1.0. A Z-score cut-off of +2 should be used to classify high weight-for-height for estimating the prevalence of overweight or obesity (also a form of malnutrition). A Z-score of -2 corresponds to the 2.3rd percentile on the reference distribution, while a Z-score of 2 corresponds to the 97.7th percentile on the reference distribution. Thus, with any of the indicators, a prevalence less than or equal to 2.3% is regarded as the surveyed population being free from malnutrition based on that indicator. Height-for-age: A low height-for-age indicates growth stunting, which reflects a long term deficit of nutritional status and/or a history of illness and disease such as diarrhea and acute respiratory infection. On a population level, a high prevalence of stunting is usually associated with poor socioeconomic conditions and a greater risk for frequent and/or early exposure to adverse environmental conditions such as illness and inadequate nutrition. A decrease in the prevalence of stunting usually parallels improvements in economic conditions. In developing countries the prevalence of low height-for-age ranges from 10% to 60%. Countries with a <20% prevalence in low height-for-age (Z-score <-2) are classified as countries with low prevalence of stunting by WHO. Weight-for-age: This index is a composite of height-for-age and weight-for-height. On a cross-sectional basis, weight-for-age is less useful than height-for-age or weight-forheight in defining nutritional status. In most populations where there are few children with low weight-for-height, the weight-for-age status provides essentially the same information as height-for-age. 22 PNG National Micronutrient Survey 2005 Weight-for-height: Low weight-for-height, or wasting, is an indicator of acute undernutrition and is often the result of severe food shortages and/or prolonged severe illness. Unlike the wide variation in stunting rates observed in developing countries, the prevalence of wasting is usually less than 5% in most countries provided there is no severe food shortage. Therefore, a wasting prevalence of more than 5% is of concern; a prevalence of 10% to 14% is considered serious; a prevalence of 15% or higher is considered critical. Standard Deviation (SD): The S.D. of the Z-score provides information on the spread of the distribution and the quality of the anthropometric measurements done for a survey. In the reference population, the standard deviation (S.D.) of the Z-score distribution for height-for-age and weight-for-height is 1.0. A Z-score S.D. that is lower than 0.9 indicates that the distribution is more homogeneous or has less variation compared to the reference distribution. A Z-score S.D. substantially greater than >1.0 indicates that the distribution has a wider spread than the reference. A Z-score S.D. <0.80 or >1.3 is suggestive of inaccurate anthropometric measurements and /or inaccurate age information. Data Quality: During data cleaning, records with potentially erroneous data will be excluded from analysis based on the following standard Z-score cutoffs developed by WHO (WHO, 1995): - height-for-age Z-score (HAZ) <-5.0 or >3.0 - weight-for-age Z-score (WAZ) <-5.0 or >5.0 - weight-for height Z-score (WHZ) <-4.0 or >5.0 Box 2. BODY MASS INDEX Adult nutritional status is assessed by calculating the Body Mass Index (BMI) from the weight and height of non-pregnant women included in the survey (BMI= Weight (kg)/Height2 (m)). A BMI below 18.5 indicates underweight or thinness. A BMI greater than 25.0 indicates overweight which can also be categorized by grade as follows (WHO, 1995). Although a BMI < 18.5 is considered underweight a BMI of 17 or < is considered a more valid cut off point for chronic energy deficiency. Underweight <18.5 Normal weight - 18.5-24.9 Overweight - 25-29.9 Obesity 30 23 PNG National Micronutrient Survey 2005 5.4 Anemia Anemia will be evaluated by photometric method using the HemoCue Hemoglobin system on small blood samples collected by finger stick. This method has shown satisfactory accuracy and precision in laboratory evaluations using standard methods. A major advantage of the battery-operated HemoCue photometer is that it readily displays hemoglobin levels with a delay of less than one minute and avoids complicated handling of blood samples in harsh field conditions. Cut-offs for anemia depends on the age and sex of the person. Anemia is defined as follows: Age-sex group Definition of anemia Children 0-59 months of age < 11g/dL Children 6-12 years of age <11.5 g/dL Non-pregnant women of child-bearing age <12.0 g/dL Pregnant women <11.0 g/dL Adult men <13.0 g/dL (WHO 2001) For prevalence estimates of anemia based on hemoglobin, the hemoglobin values will need to be adjusted for altitude (CDC, Morbidity and Mortality Weekly Report, 1998). The adjustment for altitude is shown below. Increase in cut-off Altitude (meters) point defining anemia (g/dL) < 1000 0 1000-1499 0.2 1500-1999 0.5 2000-2499 0.6 5.5 Iron status and Iron deficiency anemia Dried blood spots (DBS) will be used to analyze TfR, CRP, AGP and retinol content. Using DBS specimens offers substantial advantages in collection and handling of compared with serum and plasma in PNG, where maintaining a cold chain for serum 24 PNG National Micronutrient Survey 2005 specimens would be almost impossible. Moreover, collection of DBS specimens avoids venipuncture which may be difficult in field settings. Iron deficiency will be defined using transferring receptor (TfR). For all target groups, iron deficiency is defined as a TfR value >8.3 μg/mL1. Iron deficiency anemia will be defined as elevated TfR (>8.3 μg/mL) together with hemoglobin value below the appropriate group-specific cut off for anemia. 5.6 Vitamin A status A serum retinol concentration less than <20 µg/dL (0.70 µmol/L).0.35 mol/L defines vitamin A deficiency (VAD). The rate of VAD among preschool-age children as an indicator of VAD has been raised to >15% % in young children (<6 y)2. Serum retinol levels of 0.35 – 0.69 mol/lL (10-19 ug/dL) indicate vitamin A deficiency disorders (VADD)3. The term VADD was introduced to cover all physiological disturbances caused by low vitamin A status, including clinical signs and symptoms. The most vulnerable groups to VADD are infants, young children and pregnant and lactating women. Because retinol-RBP are decreased during bacterial infections, we will interpret retinol levels using an acute phase indicator (CRP) according to Lancet 2003; 362: 2052–58 as follows : If CRP is elevated, adjust retinol values upwards by 25% (evidence of recent infection) Or use the retinol values of only those individuals classified as healthy a the time of the survey (normal CRP) in calculating the prevalence rates of VAD and VADD Retinol data will be presented both ways. However, acute phase protein response may be impaired in severely undernourished persons. This will be considered in the data analysis. 5.7 Iodine status Urinary iodine values are expressed as micro grams per deciliter (g/dl). Levels of urinary iodine within an individual vary daily and even during a given day. Consequently, classification of iodine deficiency as a public health problem is done on the basis of median values in population groups rather than as a prevalence rate, as with vitamin A deficiency and anemia. A median urinary iodine value of less than 10.0 g/dl defines 1 Human Transferrin Receptor: An in vitro enzyme immunoassay. Ramco Laboratories, Inc. kit insert. Catalog Number TF-94. 2 Sommer A and Davidson FR, Assessment and control of Vitamin A deficiency: The Annecy Accords, J Nutr 132: 2845S-2850S, 2002 3 WHO. Indicators for assessing vitamin A deficiency and their application in monitoring and evaluating intervention programmes. 1996 25 PNG National Micronutrient Survey 2005 iodine deficiency as a problem in a population. Table5. Epidemiological criteria for assessing iodine nutrition based on median urinary iodine levels in school aged children.4 Level of importance as a public health problem Median value (g/dl) Excessive iodine (risk of adverse health consequences) > 30.0 More than adequate (risk of iodine-induced hyperthyroidism within 510 years after introduction of iodized salt in susceptible groups) 20.0-29.9 Adequate (optimal) 10.0 – 19.9 Insufficient (Mild iodine deficiency) 5.0 – 9.9 Insufficient (Moderate iodine deficiency) 2.0 – 4.9 Insufficient (Severe iodine deficieny) < 2.0 5.8 Malaria Plasmodium falciparum is the most common cause of malaria infection in PNG. All women and children participating in the survey, whether febrile or not, will have thick smears made from capillary blood samples collected after the same fingerstick used to obtain blood for hemoglobin measurement. Presence of P. falciparum trophozoites, the asexual erthrocytic development stage of the parasite, and the level of parasitemia will be recorded. Presence of P. falciparum gametocytes, the sexual erythrocytic stage and the level of parasitemia will also be recorded. Infections with Plasmodium malaraie and Plasmodium vivax are also found in PNG. If possible, microscopy will be used to differentiate among the three Plasmodium species. 5.9 Hookworm The prevalence of hookworm will be measured for each child between 24-59 months of age. The hookworm results will also be analyzed with the hemoglobin data to assess the correlation between hookworm infection and anemia. 6. Specimen Processing 4 The values in the table are for school aged children. A forthcoming WHO consultation will hopefully give more guidance as to how to interpret values in women. 26 PNG National Micronutrient Survey 2005 6.1 Hemoglobin cuvettes Hemoglobin will be assessed in the household using the HemoCue. Quality control of the HemoCue instrument will be ensured by using the control cuvette for each specific instrument before the instrument is used each. Liquid controls (HemoCue AB, Angelholm, Sweden) will also used at the beginning and end of each day as further assurance of the quality of HemoCue readings in areas where a cold chain will be kept. Log sheets of all the quality control measurements will be kept. For most PSUs where there is no cold chain the control cuvette will be used for quality assurance purposes 6.2 DBS processing At each household, fresh DBS specimens will be put aside to dry. During travel between households, DBS specimens will be placed in a microscopy slide box for protection from light and insects. In the evenings the survey team will pack the dry DBS filter papers between weighing papers and put them in low gas permeable bags with desiccant packs and a humidity monitor card. These bags will then be stored in an opaque bag to reduce light exposure. Because the buildup of humidity can damage the quality of the sample, survey team members will check the humidity monitor card in each bag of stored DBS specimens each evening and replace the dessicant packs if the card shows any color changes. 6.3 Malaria slides After the HemoCue cuvette has been filled and the DBS specimen obtained, the remaining drop of blood will be smeared onto a microscopy slide for later microscopic inspection for malaria infection. At the household, the malaria slide will be left horizontally to dry before it is inserted into the slide box for transport and storage. 6.4 Stool After a stool specimen is collected in a larger cup, one cubic centimeter of stool will be transferred from the collection cup to the stool collection tube containing 9 cc of polyvinyl alcohol (PVA). The stool will be vigorously agitated in order to mix the specimen with the PVA after the cap is screwed on and tightly sealed with parafilm. 6.5 Urine Urine samples will be collected in screw top urine collection cups. Each evening, survey team members will transfer at least 1.5 mL urine sample to each of the 2 pre-labeled cryovials using disposable pipettes. The cryovials will not be overfilled (no more than 1.8 mL) and sealed by screwing the caps on tightly The pipette and urine collection cups will be discarded in the biohazard bag. 6.6 Salt 15 grams of salt will be collected from the house. Salt should be well mixed prior to collecting the sample. The salt should be put in a pre-labeled plastic bag. 27 PNG National Micronutrient Survey 2005 7. Lab Procedures 7.1 TfR and CRP TfR and CRP will be analyzed on the DBS specimens by the staff at SEAMEO TROPMED Regional Center for Community Nutrition (RCCN) in Jakarta using a newly developed EIA method. A subset of the DBS specimens will be sent to the micronutrient laboratory at CDC and to the laboratory at Craft Technologies to be analyzed for TfR and CRP. 7.2 Retinol The vitamin A DBS will be sent to Craft Technologies for assessment using HPLC with UV/visible spectrophotometry. A sub sample will also be analyzed at UPNG (serum) and CDC (serum and DBS) using similar methods. 7.3 Malaria UPNG will perform the malaria analysis by standard microscopy with results expressed as a semi-quantitative parasite load. 7.4 Hookworm Stool samples will be assessed using centrifugation concentration and microscopy. 7.5 Urinary iodine Urine samples will be analyzed for urinary iodine at UPNG using the ammonium persulfate digestion with spectrophotometric detection of the Sandell-Kolthoff reaction. In order to assure the quality of the method to assess urinary iodine levels the laboratory, UPNG has enrolled in CDC’s quality assurance program EQUIP and in the Institute of Clinical Pathology and Medical Research (ICPMR)’s Urinary Iodine Quality Assurance Program. The laboratory at UPNG is also working with CDC on its internal quality control program. 7.8 Salt Samples will be collected and transported to Port Moresby to the UPNG laboratory for salt iodine analysis using the WYD Iodine Checker, which quantitatively measures the salt iodine content on the basis of a colorimetric method. Adequately iodized salt at the household level must contain >15 ppm iodine (ICCIDD, UNICEF, and WHO, 2001). Prior to analysis of survey salt samples, UPNG will establish an internal QC program for salt iodine testing and also participate in an EQA program for salt iodine. EQA programs for salt iodine include one managed the China National Reference Laboratory and a second run by INCAP in Guatemala. 28 PNG National Micronutrient Survey 2005 8. Data entry and analysis plan During the survey, questionnaires will be collected and entered into computer files using EpiInfo. Questionnaires will be sent to a central office in Port Moresby where the data will be entered. Any errors in completing questionnaires will be corrected in the field when possible. The team leader will be responsible for checking the data collection forms in before leaving the household. To reduce computer data entry error, the entry screen will be programmed to accept only codes within a predetermined range for most variables. All data will also be double entered and verified at the central location. Once the data is entered and the data sets are clean, the analysis of the questionnaire and the FRAT will be done by the national survey coordinator and CDC staff. Results of the biochemical indicators will be added to the analysis once the data are returned from the various laboratories (CDC, UPNG, Craft Technologies, SEAMEO TROPMED Regional Center for Community Nutrition (RCCN). Please see appendix 3 for a description of the provisions for protecting the privacy and confidentiality of participants. 9. Dissemination of study results Dissemination of study results will take place in four ways: Detailed study report: for professionals working in the field of nutrition and health at the academic and policy levels. This report will include all the details of the study, including detailed background, methodology, results and interpretation of the results. This report will be needed for a better understanding of the prevalence and causes of micronutrient deficiencies in PNG and will be used for the development of adequate national policies and programs. Easy to read summaries: for people interested in nutrition and health at international, national and district level. These summaries will mainly focus on the results and interpretation of the resultsand will be available as national and regional data. Although each of the provinces in the country will be included in the survey the precision will besufficient to interpret the results at only national and regional levels. The summaries will provide a better understanding of the causes and consequences of micronutrient deficiencies in PNG. To increase readability, this document will convey the results of the PNGNMS using mainly graphs and other visuals with minimal text 29 PNG National Micronutrient Survey 2005 Press briefing kit: for journalists and others responsible for media communications. This kit will assist journalist in informing the population of Papua New Guinea of the results and significance of the PNGNMS. This press briefing kit will include background information on micronutrient deficiencies (including copies of videos developed by UNICEF/MI), a summary of the study results, an explanation of the consequences of the micronutrient deficiencies discovered in the population of Papua New Guinea The kit will also include materials directed to specific segments within the population, such as women and children (see also number 2. above). High level advocacy meeting: for major policy makers. The objective of this meeting is to disseminate the results and to create awareness on micronutrient deficiencies, its causes and required programmatic responses among those that are responsible for implementing in place policies and programs. Examples of such persons include highranking Government officials, management personnel of companies producing fortifiable foods, and representatives of major donor organizations and countries. This one day meeting should be followed by a inter-sectoral program planning meeting to work out the recommendations made at the advocacy meeting and plan the programmatic follow up (supplementation, fortification, dietary diversification and health interventions). 10. Time frame A detailed breakdown of the survey timeline can be found in annex 4. 30 PNG National Micronutrient Survey 2005 Appendix 1: List of publications by principal investigators Principal investigators include: Gilbert Hiawayler, Victor Temple, Florence Addo, Wila Saweri, Bradley Woodruff, Katie Tripp, Rosemary Schleicher, Tracy Dearth Wesley and Bridgette Bowen. Florence Addo Gilbert Hiawayler Wila Saweri Saweri W. 2001.The rocky road from roots to rice. A review of the changing food situation in Papua New Guinea. Papua New Guinea Medical Journal; 44 (3-4): 151-163. Temu PI and Saweri W. 2001. Nutrition in transition. In: Bourke R M, Allen M G and Salisbury J G, ed. Food security for Papua New Guinea, Proceedings of the Papua New Guinea Food and Nutrition 2000 Conference, PNG University of Technology, Lae, 26-30 June 2000. ACIAR Proceedings No 99. Australian Centre for International Agricultural Research, Canberra, Australia: 395-406. Mittendorfer E and Saweri W. 1999. Nutrition country profile of Papua New Guinea. www.fao.org. FAO, Rome Saweri W. 1998. Development of health promoting schools in Papua New Guinea. Australian Journal of Nutrition and Dietetics; 55 [I Supplement]: S45-S47. Friesen H, Vince J, Boas P, Danaya R, Mokela D, Ogle G, Asuo P, Kemiki A, Lagani W, Rongap T, Varughese M and Saweri W. 1998. Infant feeding practices in Papua New Guinea. Annals of Tropical Paediatrics; 18: 209-215. Amoa B, Attah Johnson F and Saweri W. 1997. Report on Iodine Deficiency Disorders in Huon and Memyamya Districts of Morobe Province, Papua New Guinea. Department of Health/Unicef, Waigani, Papua New Guinea. Victor Temple Temple V. J., Mapira P, Adeniyi K. O. and Sims P. “Iodine Deficiency in Papua New Guinea” Journal of Public Health (Accepted for publication: March 2005) 31 PNG National Micronutrient Survey 2005 Temple V.J. and Masta A. “Zinc in Human Health” PNG Medical Journal (In Print). Temple V. J. (2003) “Iodine Deficiency Disorders (IDD): Focus on the process and significance of monitoring in PNG” Medical Science Bulletin, 28 – 32. Temple V. J. (2003) “Zinc: The controversial trace element” Med Science Bulletin, 41 – 44. Temple, V.J.; Badamosi, E.J.; Ladeji, O.; Solomon, M. and Gowok, H.G. (1996). “Proximate chemical composition of three locally formulated complementary foods” West African Journal of Biological Sciences Vol. 5. No. 2; 134 –143. Ibrahim, L.M.; Temple, V.J.; Badamosi, E.J.; Obatomi, A. and Greeley, S. (1996). “Nutrient composition of three locally prepared weaning foods” Bioscience Research Communications Vol. 8, No. 2; 71 – 76. Temple, V.J; Agye, E.O.; Badamosi, E.J.; Olomu, N.I. and Odewumi, O. (1996). “Serum levels of some biochemical parameters in pre-school age children” West African Journal of Biological Sciences, Vol. 5, No. 1; 119 – 127. Temple. V.J.; Ighogboja, I.S. and Okonji, M. C. (1994). “Serum vitamin A and betacarotene levels in pre-school age children” J. of Tropical Paediatrics, Vol. 40; 374 –375. Published Abstracts: Mapira P, Temple VJ and Adeniyi KO (2004). Using Anthropometric Data to Assess the Nutritional Status of Children (6 – 12 yrs) in Hella Region, SHP, PNG. Medical Society of PNG 40th Annual Medical Society Symposium, Sep 2004, pp 38. Mapira P, Temple VJ and Adeniyi KO (2003). Urinary Iodine Concentration In Children (6 – 12 years) in the Hella Region (Tari and Koroba Districts) In Southern Highland Province of PNG. Medical Society of PNG 39th Annual Medical Society Symposium, Sep 2003, pp 46. Mapira P, Temple VJ, Adeniyi KO (2003). Assessment of Iodine concentration in household salt and per capita consumption of salt in the Hella Region (Tari and Koroba districts) in the Southern Highlands Province PNG. Medical Society of PNG 39th Annual Medical Society Symposium, Sep 2003, pp 44. Bradley Woodruff Dowell SF, Toco A, Sita C, Piarroux R, Duerr A, Woodruff BA. Health and nutrition in centers for unaccompanied refugee children: experience from the Rwandan refugee crisis. JAMA 1995;273:1802-1806. 32 PNG National Micronutrient Survey 2005 Hassan K, Sullivan KM, Yip R, Woodruff BA. Factors associated with anemia in refugee children. J Nutr 1997;127:2194-2198. Tomashek KM, Woodruff BA, Crawford CG, Bloland P, Mbaruku G. Randomized intervention study to determine the most effective method to treat moderate anemia in refugee children in Kigoma Region, Tanzania. Am J Trop Med Hyg 2001;64:164-171. Blanck HM, Bowman BA, Serdula MK, Khan LK, Kohn W, Woodruff BA. Angular stomatitis and B vitamin status among adolescent Bhutanese refugees living in southeastern Nepal. Amer J Clin Nutr 2002;76:430-435. Woodruff BA, Duffield A. Anthropometric assessment of nutritional status in adolescent populations in humanitarian emergencies. Eur J Clin Nutr 2002;56:1108-1118. Woodruff BA, Blanck HM, Slutsker L, Cookson ST, Larson MK, Duffield A, Bhatia R. Anemia, iron status, and vitamin A deficiency among adolescent refugees: surveys in Kenya and Nepal. (submitted for publication) Hailemeskal H, Woodruff BA, Yahmed SB. Rapid health assessment. World Health: the Magazine of the World Health Organization 1996;49(6):28. Cookson ST, Woodruff BA, Slutsker L. Prevalence of anemia and low body-mass index among adolescents 10-19 years of age in refugee camps in Dadaab District, Kenya. November 1998. Woodruff BA, Slutsker L, Cookson ST. Prevalence and causes of anemia and prevalence of low body-mass index in adolescents 10-19 years of age in Kakuma camp, Kenya. April 1999. Woodruff BA, Duffield A, Blanck H, Larson MK, Pahari S, Bhatia R. Prevalence of low body mass index and specific micronutrient deficiencies in adolescents 10-19 years of age in Bhutanese refugee camps, Nepal, October 1999. Woodruff BA. Older people, nutrition, and emergencies in Ethiopia - commentary. Field Exchange 2001;14:28. Woodruff BA. Measuring mortality rates in cross-sectional surveys: a commentary. Field Exchange 2002;17:16. 33 PNG National Micronutrient Survey 2005 Woodruff BA, Reynolds M, Tchibindat F, Ahimana C. Nutrition and Health Survey: Badghis Province, Afghanistan. February – March 2002. Katie Tripp Tracy Dearth-Wesley Bridgette Bowden Rosemary Schleicher 34 PNG National Micronutrient Survey 2005 35 PNG National Micronutrient Survey 2005 Appendix 2: Sample Size Breakdown Target group Indicator Estimate Stratumd specific prevalenc half CI e Sample DEFF size per stratum if SRS Sample size per stratum if cluster sampling Sample Individual size needed (all nonresponse 4 strata) No. per HH Non Total number response HH incl. nonfor HH response Total number of HH for 4 strata Specimens if sample size = 1600 HH Difference between actual & needed sample size HH Salt HH 0.5 0.12 67 4.5 300 1201 0% 1 10% 333 1,334 1440 240 0.5 0.10 96 2 192 768 20% 0.7 10% 381 1,524 806 38 0.5 0.10 96 2 192 768 20% 0.7 10% 381 1,524 806 38 Malaria 0.5 0.12 67 3 200 800 20% 0.7 10% 397 1,588 806 6 Vitamin A deficiency 0.5 0.10 96 2 192 768 20% 0.7 10% 381 1,524 806 38 Wasting 0.1 0.05 138 1.5 207 830 10% 0.7 10% 366 1,463 907 77 Stunting 0.5 0.10 96 1.5 144 576 10% 0.7 10% 254 1,016 907 331 Hookworm 0.5 0.15 43 3 128 512 30% 0.5 10% 407 1,626 504 -8 0.5 0.10 96 2 192 768 20% 1.37 10% 195 779 789 21 Anemia 0.5 0.10 96 2 192 768 20% 1.37 10% 195 779 789 21 Malaria 0.5 0.12 67 3 200 800 20% 1.37 10% 203 811 789 -11 BMI <17 0.1 0.05 138 1.5 207 830 10% 1.37 10% 187 748 888 58 BMI >25 0.5 0.10 96 3 288 1152 10% 1.37 10% 260 1,039 888 -265 Urinary 0.5 0.10 96 2 192 768 20% 1.37 10% 195 779 789 21 Anemia 0.1 0.05 138 1.5 207 830 25% 1.5 10% 205 820 810 -20 BMI < 17 0.1 0.05 138 1.5 207 830 10% 1.5 10% 171 683 972 142 BMI > 25 0.1 0.05 138 1.5 207 830 10% 1.5 10% 171 683 972 142 Children 6-59 Anemia months Iron deficiency Children 2459 months Women 15-49 Iron years deficiency Iodine Men > 18 years 36 PNG National Micronutrient Survey 2005 Appendix 3: Supplemental information for protection of human research participants Description of risks (physical, social, psychological) to the individual The only physical risk of participation in nutrition assessment surveys includes only those of fingerstick and phlebotomy. Both procedures involve some discomfort but there are only very rarely any adverse risks associated. Only qualified survey team members are allowed to perform these procedures, and these personnel monitor survey participants for some minutes after the procedure to ensure that there is no bleeding, serious bruising, or vasovagal syncope. Social and psychological risks are minimal. No sensitive questions will be asked during interviews. Although in many communities, privacy is difficult to achieve during data collection, all possible efforts will be made to keep participants responses and findings as private as possible. No survey data will ever be connected to identifying information in a report, manuscript, or verbal debriefing. Anticipated benefits of participating in the survey As mentioned above, participants will learn the results of the hemoglobin and may benefit from referral. In addition, indirect benefits to survey participants include the benefits accrued to the entire population as a result of the interventions based on the survey findings. Description of the potential risks to anticipated benefit ratio The risk of participation includes only the minimal risk of finger stick and phlebotomy for a sub sample of the survey participants. The benefit includes the implementation of critical food and nutrition programs in population with potentially serious nutritional deficiencies. Justification for involving vulnerable participant populations Protein-energy malnutrition and most micronutrient deficiencies occur in vulnerable populations, such as young children and women of child-bearing age. As a result, assessment of these problems must be directed to these vulnerable populations to achieve the maximum likelihood of detecting these conditions if they are present in the population. Procedures for implementing and documenting informed consent Prior to enrollment, verbal consent will be obtained from all adults and from parents or caretakers of children selected for participation in the survey. The explanation of the survey will include the purpose of the survey, the types of questions which will be asked, 37 PNG National Micronutrient Survey 2005 the procedure for weighing and measuring children 6-59 months of age and women of child-bearing age and a description of the type and quantity of biologic specimens to be obtained as well as the procedures necessary for specimen collection. The granting of verbal consent will be indicated on the data collection form. Written consent is not practical in most circumstances due to high rates of illiteracy, the risks to survey participants are very small, and the time necessary for extensive explanation of all facets of the survey is not available. Survey team members will advise survey coordinators regarding the culturally most appropriate way to explain the survey and obtain verbal consent. Procedures for implementing and documenting the assent process of children Because the children included in most nutrition assessment surveys are less than 5 years of age, assent is not required. Consent will be obtained from the mothers or guardians of all children in the survey. Provisions for protecting privacy/confidentiality The paper data collection forms completed in the field will not contain identifying information. As a result, the computer dataset resulting from data entry of these forms will also not contain any identifying information. Data collection forms and laboratory specimens are matched by recording on both forms and specimens a numeric code number which is specific for each household and individual participant. In this survey a sample list, which lists all the households or individuals selected for that cluster (cluster control form) will be created when the final selection of households or individuals is made during the sampling process. These cluster control forms will contain both identifying information and the numeric code described above, and thus can serve as a key to match the code numbers to specific individual participants. At the end of the survey, these sample lists will be destroyed or permanently stored separately from the paper data collection forms. Only authorized survey coordinators will have access to the sample lists and data collection forms. 38 PNG National Micronutrient Survey 2005 Appendix 4: Time Frame Task Jul Aug Sep Oct Nov Dec Jan Feb Mar Apr May Jun Jul Debrief UNICEF-PNG Debrief UNICEF-EAPRO and INMU Calculate final sample size (1600) Create list of equipment and supplies Select clusters Identify and engage survey manager Prepare final budget Check with Neil Craft and Juergen Erhard re laboratory testing Identify lab person at CDC to travel to PNG Run EQUIP at PNG laboratory Discussion with INCAP Establish quality assurance Discuss potential survey workers Negotiate for cheap flights and other transport Investigate cost of anthropometric equipment Funds arrive Order supplies and equipment Identify potential training facilities Develop data collection forms Translate and backtranslate questionnaire questions? 39 Aug Sep PNG National Micronutrient Survey 2005 Develop social mobilization plan Request authorization to import supplies and equipment Discuss data entry and analysis with NSO and IMR Pre-test questionnaire questions Submit protocol to IMR for ethical clearance (Nov 11) Finalize list of survey workers and invite them to training Develop social mobilization materials Organize supplies for pilot Start to organize training Check status of supplies and equipment !!! Plan training curriculum Develop survey manual Identify trainers Identify expert supervisors for first few clusters Identify secretariat for training Check status of supplies and equipment !!! Pilot test procedures Check status of supplies and equipment !!! Develop data entry system Plan training, incl standardization, facility, accomodation of trainees, etc. Begin preparation of report 40 PNG National Micronutrient Survey 2005 Ensure ALL supplies and equipment have arrived and are functioning !!! Training (week 2 or 3 of March) Training for data entry Data collection ? Data entry Laboratory specimen analysis Data cleaning Data analysis Submit final report Workshop to discuss results 41 PNG National Micronutrient Survey 2005 Appendix 5 Measurement of Micronutrients in Dried Blood and Serum Spots Validation in Potentially Deficient Populations Background The concentrations of vitamin A (retinol), ferritin, soluble transferrin receptor (TfR), and C-reactive protein (CRP) circulating in serum are commonly used parameters for assessing vitamin A and iron status. Preparing and transporting serum for these tests is not a trivial issue in developing countries. Access to centrifuges, refrigerators, freezers, cold packs and dry ice is often impractical. There remains a critical need for simple and affordable laboratory methods for assessment of micronutrient status in populations at risk for deficiency. Dried blood spots (DBS), collected and processed in the field and tested at distant laboratories, offer a simple and affordable method for obtaining and transporting specimens. DBS are currently being used by one commercial lab in the United States for nutritional testing, Craft Technologies (Wilson, NC). This lab has a strong track record in the development and use of methodologies for assessing micronutrient status in deficient populations. The major advantages of DBS are 1) elimination of the need for venous blood sampling, which is often a technical challenge in infants and young children (typically DBS tests require smaller quantities of blood than serum-based tests), and 2) convenience and low cost transportation of specimens on filter paper blood collection devices at ambient temperature. A Technical Report (1) reviewing the quality, completeness, analytical approach, and results of the process followed for field validation of the Craft Technologies vitamin A DBS method, particularly within the low range commonly found in populations at risk of vitamin A deficiency, noted that there are no data on the decomposition rate of blood spot retinol in children when their serum retinol concentrations are less than 1.05 µM (30 µg/dL). The report suggested that the Craft method needs to be evaluated by a third-party laboratory that has no financial interest in the outcome. They also suggested that the DBS methodology must be tested in three to five distinct “real world” settings. Further research into the utility and reproducibility of the DBS methodology in different countries is critically needed. Research needs include the intra-individual variation, ramifications of the poorly collected DBS, data about the volume of serum that is needed for analysis, and the effect of freeze-thaw cycles on results. These and other questions can be raised concerning the use of DBS for other markers of nutrition (or infection) status such as ferritin, retinol binding protein (RBP), TfR, alpha1-acid glycoprotein (AGP) and CRP. Few of the DBS assays for these markers have been well validated in the field. A dried serum spot (DSS) assay for ferritin (2) offers the advantage of shipping samples at ambient temperature, and fulfills WHO recommendations for iron status testing (May, 2004 meeting not yet published), but has not been widely used in the field. A portable centrifuge would still be required for preparing serum, but transportation of specimens to the lab is simplified. This method similarly needs to be evaluated under field conditions by others. 42 PNG National Micronutrient Survey 2005 CDC is often involved in nutrition assessment surveys in developing countries. We are often asked whether the DBS methods are valid. Typically we review the literature and contact labs that perform such assays to request validation information. Often validation information is not available, or is not comprehensive enough to evaluate the methodology. The overall objective of this protocol is to perform ancillary research based on non-research nutrition assessments in countries performing national nutrition surveys. We foresee that we might have the opportunity to perform a direct comparison of conventional serum testing versus dried blood spot and dried serum spot testing 1-3 times per year. The nutrition assessments are non-research public health need surveys undertaken with the goal to make recommendations about nutrition policy. The populations involved in these surveys generally suffer multiple micronutrient deficiencies. Small method validation research projects would be performed using convenience samples from the nutrition surveys. The participants would either be part of the survey, members of the survey household not directly included in the survey, or field staff who volunteer for this research. We plan to take capillary and venous blood, and prepare dried blood spot and dried serum spot samples from persons at locations within the countries where access to a centrifuge, freezer and dry ice is readily available. Each additional study after the first one, which is detailed in this protocol, would be written up as an amendment to this protocol using CDC IRB form 1252. We anticipate collecting up to 200 pairs of serum-DBS-DSS samples in each of 3-4 regions around the world in populations with varying degrees of micronutrient malnutrition. It will likely take 1-2 years to collect this information. We would comparatively test for as many nutritional indicators as possible including vitamin A, retinol binding protein (RBP), ferritin, TfR, AGP and CRP. DBS RBP which is the carrier protein for vitamin A will be compared with vitamin A and a new RBP kit assay. DBS AGP data will be compared with CRP data (infection markers), although the results are not expected to be well-matched as the time course differs for each marker depending on the course of the infection. If possible, the CDC lab will develop a test for AGP in serum. In some studies we would include whole blood zinc protoporphyrin to confirm iron deficiency using an independent test. The ZP test requires one drop (20 uL) of whole blood. In some studies we would exchange samples with labs that are performing DBS or DSS assays to compare results and provide quality assurance information for the nutrition survey. The amendments would specify such details about the particular study. Assurances and informed consent forms would be included at the time each amendment is proposed. Protocol Details The first study will take place in Papua New Guinea (PNG) in Spring, 2005. Subjects will be selected on the basis of whether they are willing and able to donate a venous sample (<30 cc = 2 tablespoons) and meet the following age and gender criteria – Group Gender Age EDTA Whole Blood Serum Finger stick Children ♂ or ♀ 24-59 months venous (<15 cc) venous blood (<15 cc) 4-5 drops Adult female 18-49 years venous (<15 cc) venous blood (<15 cc) 4-5 drops Adult male > 18 years venous (<15 cc) venous blood (<15 cc) 4-5 drops 43 PNG National Micronutrient Survey 2005 Analtyes will be tested at the following locations – Analyte Matrix Lab AGP DBS University of Indonesia CRP serum CDC Nutrition Lab CRP DBS Craft Technologies CRP DBS University of Indonesia Ferritin serum CDC Nutrition Lab Ferritin DSS CDC Nutrition Lab Ferritin DSS Penn State University RBP serum CDC Nutrition Lab RBP DBS CDC Nutrition Lab RBP DBS University of Indonesia TfR serum CDC Nutrition Lab TfR DBS CDC Newborn Screening Lab TfR DBS Craft Technologies TfR DBS University of Indonesia VIA serum CDC Nutrition Lab VIA serum University of PNG VIA DBS CDC Nutrition Lab VIA DBS Craft Technologies ZP blood University of PNG Finger stick blood will be collected in 4 blood spots to simulate the blood collection method that will be used in the national survey in PNG. Each tube of whole blood (~15 mL) will be sufficient to prepare approximately 600 blood spots (25 uL/spot). Each tube of serum (~ 7 mL) will be enough for at least 50 tests on serum and 20 DSS (100 uL/spot) specimens. Sufficient numbers of samples are needed for comparison of intra- and inter-day variability within and between labs. Serum aliquots and dried blood and dried serum spots will be prepared in PNG. With one exception, dried cards and serum will be shipped to CDC for repackaging and distribution. The University of PNG serum aliquots will be transported directly to the lab from the survey collection without shipment to the US. Study subjects will be identified with the help of the PNG nutrition survey workers. The estimated number of participants will range from 150-200 subjects. Subject information connected to the samples will be limited to gender, age and hemoglobin. Hemoglobin information is necessary for selecting calibration spots for the DBS assays. Trained medical or laboratory personnel will collect clinical samples using standard protocols for specimen collection, handling, storage, and shipment to CDC for further distribution with the exception of ZP testing which will be done each day at the University of PNG. Survey staff will use standardized consent forms to obtain consent 44 PNG National Micronutrient Survey 2005 from adults and from the parents of children younger than 18 years of age (see attachment). Standardized assent forms will be used to obtain assent to participate from children less than 18 years old (see attachment). A zinc protoporphyrin (ZP) test will be used to independently evaluate iron status. This test requires 1 drop of blood. The rationale for this test is that WHO guidelines (May, 2004 meeting not yet published) found insufficient information to recommend this test for iron status. From a field work point of view, this test is simple, cheap and the instrument can be used in-country using a 12 volt battery. Although not part of the serum-DBS-DSS comparison, this information will add great value to this research project. The results from the DBS, DSS and serum for iron indicators (TfR and ferritin) will be compared with ZP results. Field data will be accumulated with each research protocol. In the event of unexpected findings for any indicator, CDC will immediately relate this information to the community via the PNG Nutrition Survey Coordinators located at MOH, UNICEF and the University of PNG, and through contact with the University of PNG and CDC Institutional Review Boards. References 1. Dried blood spot retinol and retinol-binding protein concentrations using enzyme immunoassay as surrogates of serum retinol concentrations. Sherry A. Tanumihardjo, William S. Blaner, Tianan Jiang. MOST Technical Report, June 2002 2. Ahluwalia N, de Silva A, Atukorala S, Weaver V, Molls R. Ferritin concentrations in dried serum spots from capillary and venous blood in children in Sri Lanka: a validation study. Am J Clin Nutr. 2002 Feb;75(2):289-94. 45