Determination of Maximum Residue Levels (MRL)
advertisement

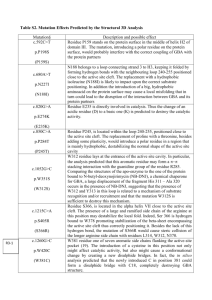
DETERMINATION OF MAXIMUM RESIDUE LEVELS (MRL) AND ANALYTICAL REQUIREMENTS Stefan Soback Kimron Veterinary Institute PO Box 12 Beit Dagan 50250,Israel Tel. +972 3 968 1713; Fax +972 3 968 8936 E-mail stefans@moag.gov.il Maximum residue levels (MRLs) in edible animal tissues are used to ensure consumer safety, to facilitate fair international trade and to allow prudent use of veterinary drugs in animals. A Maximum Residue Limit is a level of residue that could safely remain in the tissue or food product derived from a food-producing animal that has been treated with a veterinary drug. This residue is considered to pose no adverse health effects if ingested daily by humans over a lifetime. The Acceptable Daily Intake (ADI) is determined on toxicological or microbiological (for antimicrobials) end-point determination by use of a No Observed Effect Level (NOEL). Based on the dietary consumption levels the ADI is allocated to species and tissue specific MRLs. In addition to the toxicological and microbiological the analytical method performance has a central role in MRL allocation. No MRL can be allocated unless there is an analytical method allowing the enforcement of that level. Presently a wide range of analytical techniques are accepted including microbiological, immunological and chemical assays. There are certain basic requirements for the assay procedures. Because the MRL is a concentration, the method must be able to measure the concentration at the level (concentration) generated from the ADI allocation. There are requirements for the accuracy of the determination. The MRL is also a compound dependent entity, which requires assay specificity. Some of the assays can not meet the requirements of one or both of the above criteria (accuracy/specificity). Such assays may therefore be applicable only for screening purposes. This class generally incorporates bioassays and immunoassays. Such results need to be confirmed with specific chemical assay providing both the identification and the quantification of the chemical entity. The modern chemical assays include chromatographic methods incorporating a wide variety of detectors. The official methods usually take into account also the availability of the analytical instrumentation in a regulatory laboratory. However, modern GC/MS and above all LC/MS/MS methods are becoming standard methods in the routine residue control and monitoring programs. Organized and Produced: http://www.isranalytica.org.il P.O.B 4034 Ness-Ziona 70400 Tel.+972-8-940-9085, Fax. .+972-8-940-9086 Site: www.BioForum.org.il E-mail: BioForum@bezeqint.net