Full Article
advertisement

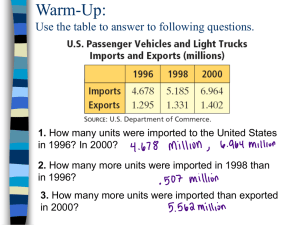
244 FARMACIA, 2008, Vol.LVI, 3 THE INFLUENCE OF SOME HYDROPHILLIC POLYMERS UPON DILTIAZEM HYDROCHLORIDE DISSOLUTION FROM MATRIX DOSAGE FORMS FORMULATION CAMELIA BALAZS1*, SORIN E. LEUCUTA2 Europharm S.A. Braşov University of Medicine and Pharmacy "Iuliu Hatieganu" Cluj-Napoca, 13, Emil Isac Str., Cluj-Napoca, Romania, Department of Pharmaceutical Technology and Biopharmaceutics *corresponding author: balazs_camelia2006@yahoo.com 1 2 Abstract Matrix tablets containing diltiazem hydrochloride were prepared by the direct compression method, using hydrophilic polymers, hydroxypropyl methylcellulose (HPMC, type Methocel K15M), hydroxypropyl cellulose (HPC, type Klucel HXF) and polyethylene oxide (PEO, type Polyox 1105). Diltiazem hydrochloride dissolution from matrix tablets was in accordance with the hydrophilic polymer type and level. Hence, it was obtained a slow release for diltiazem. The in vitro release of diltiazem was determined using UV spectrophotometry at 237 nm. Results, in conformity with the requirements of one dissolution test for diltiazem, from U.S.P., can be obtained with formulations based on PEO. The diltiazem release mechanism from hydrophilic matrices was observed by correlation with kinetic models. Rezumat Comprimate matriceale conţinând clorhidrat de diltiazem au fost preparate prin metoda comprimării directe, utilizând polimeri hidrofili, hidroxipropilmetilceluloza (HPMC, tip Methocel K15M), hidroxipropilceluloza (HPC, tip Klucel HXF) şi polietilenoxid (PEO, tip Polyox 1105). Dizolvarea clorhidratului de diltiazem din comprimatele tip matriţă a fost în funcţie de tipul şi nivelul polimerului hidrofil în formulare, obţinând viteză redusă de dizolvare. Cedarea in vitro a diltiazemului a fost determinată spectrofotometric UV la lungimea de undă de 237 nm. Rezultate, în conformitate cu prevederile unui test de dizolvare pentru diltiazem din farmacopeea S.U.A., se pot obţine cu formulări pe baza de PEO. Mecanismul de cedare al diltiazemului din matriţele hidrofile realizate a fost urmărit prin corelarea cu modele cinetice. Diltiazem hydrochloride Polyethylene oxide Hydrophilic matrix tablets INTRODUCTION Hypromellose (HPMC), hydroxypropylcellulose (HPC) and poly(ethylene oxide) (PEO) are water soluble polymers, available in a variety of FARMACIA, 2008, Vol.LVI, 3 245 molecular weights and the corresponding viscosity types. Some sorts, showing high viscosities in aqueous solution, are used in extended release applications of hydrophilic matrices due to their hydration and swelling capacities. This behaviour is not affected by the gastrointestinal pH, due to their polymeric non ionic structures. Swellable matrix tablets are activated by water, and drug release control depends on the interaction among water, polymer and drug. The mechanisms of drug release are diffusion of drug through the gel layer produced and drug transport due to the relaxation of the polymer. The rate of diffusion through the gel layer depends on drug dissolution and matrix erosion [6]. HPMC matrices present a continuous swelling, while PEO matrices have a quick hydration and gelation of the matrix, but generate a worse gel, for polymer types with comparable viscosity. In this way, the PEO matrix system is more susceptible of erosion process and the erosion speed is greater than HPMC matrix system [3]. Different types of swellable matrix tablets can be prepared by using hydrophilic polymers. The most common are the free swellable matrix tablets (polymer and solid drug mixed and compressed) in which swelling is unhindered. In order to introduce additional elements for the drug release control, the swelling of these matrix tablets can be affected by matrix surface modification [6]. In this way, diltiazem formulation with HPMC and PEO in Geomatrix technology leaded to a constant release. This system is based on three layer geometry, these layers delayed the initial hydration of matrix core, limited the diffusion only in the lateral side of the tablet and eroded gradually. In this way, it was obtained zero order release [5]. In the specialty literature, diltiazem with HPC formulations were found in the pulsatile drug release dosage forms, alongside with other polymers, prepared by multilayer compression techniques [2]. The objective of this study was diltiazem hydrochloride formulation with some hydrophilic polymers, with viscosity types usually used in extended release, as HPMC type Methocel K15M, HPC type Klucel HXF and PEO type Polyox 1105. There were prepared tablets by direct compression. It was studied the diltiazem hydrochloride dissolution from hydrophilic matrices resulted and the mechanism of active released. MATERIALS AND METHODS Materials The diltiazem hydrochloride powder used was from Dr. Reddy’s (India) producer. 246 FARMACIA, 2008, Vol.LVI, 3 The hydrophilic polymers used were: Methocel K15MCR produced by Dow Chemicals distributed by Colorcon; Klucel HXF, Hercules producer and Polyox 1105 Union Carbide producer. Other excipients used were: mycrocrystalline cellulose Vivapur 102 type, J. Rettenmaier&Sohne producer; colloidal silicium dioxide aerosil 200 type, Degussa producer; magnesium stearate, Magnesia producer. The molecular weights for hydrophilic agents used and the viscosity values (in cP) for aqueous solution of polymers are mentioned in table I. Polymer Methocel K15MCR Klucel HXF Polyox 1105 Table I Viscosity values for aqueous solution of polymers [3] Molecular weight Viscosity (cP) Type 2208 15,000 (2% sol) 1,150,000 2,500 (1% sol) 900,000 8,800-17,600 (5% sol) Methods 1. Hydrophilic matrices preparation There were prepared tablets with 10 mm diameter by direct compression method. The batch size was laboratory scale, 500 tablets. The diltiazem hydrochloride, polymers and excipients powders were weighed, sifted by 0.6 mm opening sieves. The powders were homogenized in a cube with 1 liter volume, at 15 rpm rotation speed for 10 minutes, in a blender type Pharmatech MB025. The compression was made using a tabletting machine with one punch, using a medium tabletting force. Three ratios diltiazem hydrochloride: polymer, 2:1, 1:1 and 1:2, were established for each of the three polymers used for producing hydrophilic matrices by direct compression. The formulation quantities for active, polymer and excipients are mentioned in table II. Table II Formulation quantities Diltiazem: polymer ratio 2:1 1:1 1:2 Diltiazem hydrochloride [mg] 120 120 120 Polymer [mg] Vivapur 102 [mg] Magnesium stearate [mg] Aerosil 200 [mg] Sum [mg] 60 120 240 239 179 59 4 4 4 2 2 2 425 425 425 247 FARMACIA, 2008, Vol.LVI, 3 2. In vitro dissolution study In vitro dissolution tests were made according to “Diltiazem Hydrochloride Extended-Release Capsules” monograph, USP 27 [1]. The conditions for the 8th test were: apparatus 2 (paddles) at 100 rpm rotation speed and 900 ml purified water at 370C as dissolution medium. The limits for diltiazem hydrochloride dissolved percentages are mentioned in table III. Table III Limits for diltiazem hydrochloride dissolved percents [1] Time (hours) 0 1 4 10 15 % dissolved 5-20% 30-50% 60-90% min 80% The prelevation times were 0.5, 1, 2, 3, 4, 6, 8 and 12 hours. Dissolution tester was Sotax AT 7 Smart. The quantity of diltiazem hydrochloride dissolved was measured by UV spectrophotometry at 237 nm. There were made three dissolution determinations for each formulation and a mean value was calculated for each dissolution result [8]. 3. Kinetics dissolution study There were used mathematic models from literature to describe the diltiazem hydrochloride release kinetics from the resulted hydrophilic matrices. The selection criterion for the suited model was the correlation of active dissolved percents with the time in a mathematic model linear equation. The correlation coefficient R value needs to be approximately 1 [6]. RESULTS AND DISCUSSION Diltiazem hydrochloride dissolution from hydrophilic matrices results The dissolution results are presented in table IV. These are values obtained at diltiazem hydrochloride dissolution from hydrophilic matrices prepared by direct compression. 248 FARMACIA, 2008, Vol.LVI, 3 Table IV Percentages of diltiazem released in different periods of time Polyox 1105 Klucel HXF Methocel 15M Diltiazem : 0.5 1 2 3 4 6 8 12 polymer hours hour hours hours hours hours hours hours 2:1 21.73 23.4 34.05 44.10 51.53 77.75 84.45 88.15 Dev std 0.147 0.326 0.488 0.652 0.817 0.637 0.731 0.147 1:1 12.82 18.14 25.67 33.26 38.19 47.2 55.42 63.20 Dev std 0.181 0.263 0.383 0.512 0.607 0.773 0.552 0.668 1:2 13.05 17.90 23.26 29.90 33.16 41.85 48.55 54.14 Dev std 0.174 0.245 0.328 0.435 0.504 0.648 0.413 0.387 2:1 19.12 21.75 30.61 39.44 43.96 93.95 94.80 95.01 Dev std 0.129 0.303 0.439 0.583 0.697 0.762 0.263 0.220 1:1 10.99 18.00 24.72 30.79 35.50 43.23 50.10 56.20 Dev std 0.155 0.261 0.369 0.360 0.564 0.708 0.362 0.289 1:2 11.47 16.14 20.50 26.72 29.95 38.22 45.68 52.30 Dev std 0.153 0.221 0.289 0.389 0.449 0.592 0.442 0.631 2:1 15.91 19.41 31.64 39.73 43.09 56.01 66.64 73.40 Dev std 0.220 0.276 0.463 0.599 0.669 0.802 0.246 0.411 1:1 9.94 17.83 26.14 34.26 40.94 51.67 61.44 70.22 Dev std 0.140 0.258 0.390 0.533 0.651 0.846 0.521 0.613 1:2 11.61 16.41 24.14 32.75 37.73 47.45 57.32 65.20 Dev std 0.155 0.225 0.341 0.476 0.566 0.735 0.413 0.512 Note: There are mean values for percents dissolved; dev std means standard deviation Diltiazem hydrochloride dissolution determined by polymer type in formulation is presented in fig. 1. The diltiazem percents dissolved are lower in the case of Polyox 1105 matrix than in Methocel K15M and Klucel HXF matrices, at the 2:1 diltiazem: polymer ratio. This is due to the gel barrier generation speed at the tablets matrices contact with the dissolution medium. The PEO matrix rapidly swells, the hydration speed is high and the gel barrier is well defined until the diltiazem diffusion starts, in comparison with HPMC and HPC matrices. The HPMC and HPC matrices present a continuous swelling in aqueous medium and the gel barrier are incompletely created at this ratio active: polymer. 249 FARMACIA, 2008, Vol.LVI, 3 100 100 Diltiazem : Polymer 2 : 1 80 70 70 60 50 40 Methocel K15M 30 20 Polyox 1105 10 hours 0 1 2 3 4 5 6 7 8 60 50 40 Methocel K15M 30 Klucel HXF 0 Diltiazem : polymer 1:1 90 80 % dissolved % dissolved 90 9 10 11 20 Klucel HXF 10 Polyox 1105 hours 0 12 0 1 2 3 4 5 6 7 8 9 10 11 12 100 Diltiazem : polymer 1:2 90 80 % dissolved 70 60 50 40 30 Methocel K15M 20 Klucel HXF 10 hours Polyox 1105 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Figure 1 Diltiazem hydrochloride dissolution: polymer type influence The diltiazem percents dissolved are lower in the case of hydrophilic matrices generated by Methocel K15M and Klucel HXF than the matrix generated by Polyox 1105, at the 1:1 and 1:2 diltiazem: polymer ratios. The dissolution results for 1:1 and 1:2 ratios are conform to literature notes. Diltiazem release from PEO matrices is more rapid and complete than diltiazem release from HPMC and HPC matrices, polymer types with comparable viscosities [7]. The reason is Polyox 1105 forms a weak gel, in comparison with Methocel K15M and Klucel HXF. This Polyox barrier is easily removed by dissolution medium and matrix system is more sensitive to the erosion phenomenon. There is a decrease, emphasized in Diltiazem tablet volume, and the erosion speed for PEO matrices at the mentioned ratios is greater than HPMC and HPC matrices. Diltiazem hydrochloride dissolution determined by polymer level in hydrophilic matrices is presented in fig. 2. The dissolution speed decreases with the increase in polymer level in formulation, for the polymers used in formulation. The diltiazem release depends on hydrophilic matrices specific factors: drug level and polymer nominal viscosity and level in formulation [6]. For a small amount of polymer, simultaneously high proportion of active in formulation, the diltiazem release is governed by matrix porosity done by diltiazem dissolution and diffusion. The polymer level increase brings to gel viscosity increasing and the active diffusion is decreased, with dissolution speed decreasing. 250 FARMACIA, 2008, Vol.LVI, 3 100 100 90 Methocel K15M 80 80 70 70 % dissolved % dissolved 90 60 50 40 Klucel HXF 60 50 40 30 2:1 30 2:1 20 1:1 20 1:1 10 10 hours 1:2 0 hours 1:2 0 0 1 2 3 4 5 6 7 8 9 10 11 12 0 1 2 3 4 5 6 7 8 9 10 11 100 90 Polyox 1105 80 % dissolved 70 60 50 40 2:1 30 1:1 20 1:2 10 hours 0 0 1 2 3 4 5 6 7 8 9 10 11 12 Figure 2 Diltiazem hydrochloride dissolution: polymer level influence Dissolution results in comparison with USP limits The study purpose was USP limits proximity for diltiazem dissolution from hydrophilic matrices prepared in this work. The limits for the 8th test are mentioned in table III. The dissolved percents value needs to be 20% maximum after the first hour, 30-50% after 4 hours, around 60% after 8 hours and 70% minimum after 12 hours. Diltiazem matrices formulation with Polyox 1105, 2:1 and 1:1 ratios diltiazem: polyox, are nearby with USP limits, 8th test, for diltiazem in vitro dissolution. Kinetics dissolution study Hydrophilic matrix performance, as prolonged release system, is dependent to: matrix water penetration, polymer swelling, substance dissolution and diffusion and matrix erosion [7]. The kinetics study was used to elucidate the physical mechanisms of drug transport by simply comparing the release data to mathematical models. Higuchi equation predicts a linear relation if Q is graphically represented in function of the square root of time, t1/2, equation (1): 12 251 FARMACIA, 2008, Vol.LVI, 3 Q ( K H .t 1 / 2 ) c, (1) where Q is the cumulative percent of drug release in time (t), KH is the Higuchi release constant (slope), c is the y axis intercept [6]. The values for correlation coefficient, R, between diltiazem dissolved percents and square root of time, were approximately 1, for each type of studied matrix, values presented in table V. Table V Correlation between diltiazem dissolved percents and time, in conformity to kinetics models, Higuchi (H) and Korsmeyer-Peppas (K-P) diltiazem : H Higuchi K-P Polymer polimer Correlation Constant Correlation n ratio coefficient Release KH coefficient Methocel 2:1 0.985 30.247 0.975 0.530 K15M 1:1 0.998 18.945 0.999 0.517 1:2 0.997 16.024 0.998 0.462 Klucel 2:1 0.911 32.449 0.928 0.578 HXF 1:1 0.997 16.916 0.996 0.512 1:2 0.998 15.391 0.997 0.488 Polyox 2:1 0.996 22.276 0.994 0.516 1105 1:1 0.996 21.659 0.996 0.617 1:2 0.997 19.852 0.998 0.566 The release mechanism described on this kinetics is by matrix pores diffusion of drug dissolved. The slope, KH, was calculated in conformity with the model equation. The KH represents drug release rate constant and is declared as dissolved percents / hours1/2 or hours-1/2. The KH values were 22.3 and 21.6 hours-1/2 for the formulations with Polyox, 2:1 and 1:1 ratios, according to USP limits, 8th test, for diltiazem dissolution (table V). A common procedure for analyzing experimental data of drug release from swelling hydrophilic systems is fitting them to the exponential equation (2) from Korsmeyer-Peppas model, and determining the exponent n. Mt K K P .t n , M ( 2) 252 FARMACIA, 2008, Vol.LVI, 3 Where Mt is the drug release quantity at time t (hours), M∞ is the total drug release quantity after a long time, Mt/M∞ is the release fraction at time t. KK-P is the release constant and n is a dimensionless number, indication exponent for the release kinetics. The n index is the right slope of graphic representation of log (normalized drug release, Mt/M∞) as a function of log (release time, t) [4, 6] The diffusion mechanism was confirmed by exponential equation from Korsmeyer-Peppas model, the correlation coefficient values were mentioned in table VI. The n index had 0.46 to 0.62 values (table VI). This model describes drug release kinetics from swellingcontrolled drug delivery systems, dependent on matrix geometry. For a cylinder matrix, 0.45 n value indicates t1/2 dependent kinetics, in accordance with Fickian diffusion, 0.45<n<0.89 values show anomalous transport, nonFickian diffusion, sum of the two mechanisms, release controlled by diffusion with relaxation / erosion of polymer [6]. Thus, the diltiazem hydrochloride release mechanism from the realized hydrophilic matrix tablets, was performed by drug diffusion on the gel layer generated by the polymer hydration and swelling. This stage was followed by the gel layer erosion. Erosion phenomenon was more visible at PEO matrices than HPMC and HPC matrices, at 1:1 and 1:2 diltiazem: polymer ratios. CONCLUSIONS Hydrophilic matrix tablets were prepared by diltiazem hydrochloride tablets formulation with different levels of water soluble and swellable polymers, hypromellose, hydroxypropylcellulose and poly(ethylene oxide), using polymer and drug powders mixing and direct compression technologies. Diltiazem release from the produced swellable matrix tablets was determined by the polymer type and the level in formulation. At the smallest level of matrix agent in formulation, 2:1 ratio diltiazem: polymer, the drug release was governed by the rapid hidration and matrix generation for PEO. At this level the diltiazem release rate for PEO matrix was lower than the rates for HPMC and HPC matrices. At higher levels of matrix agent in formulation, 1:1 and 1:2 ratios diltiazem: polymer, the drug release was determined by the higher vicosity of the gel barrier in case of HPMC and HPC, and weakly, erodible gel layer in case of PEO. At this levels, the diltiazem release rates for HPMC and HPC matrices were lower than the rate for PEO matrix. FARMACIA, 2008, Vol.LVI, 3 253 The diltiazem release rate was decreased while the polymer level increased, for all three matrix agents used in diltiazem formulation in this issue. The drug dissolved results, close to U.S.P. in vitro dissolution test limits, were the 2:1 and 1:1 diltiazem : polyox 1105 formulations. The diltiazem hydrochloride release mechanism was done by drug diffusion on the gel layer generated by the polymer hydration and swelling, followed by the gel layer erosion. Erosion phenomenon was more visible at PEO matrices than HPMC and HPC matrices, at 1:1 and 1:2 diltiazem: polymer ratios. REFERENCES 1. xxx, USP 27, United States Pharmacopoeia, 27th Edition, 2004, Diltiazem Hydrochloride Extended-Release Capsules monograph 2. Fukui E., Uemura K., Kobayashi M. “Studies on applicability of press-coated tablets using hydroxypropylcellulose (HPC) in the outer shell for timed-release preparations”, Journal of Controlled Release, 2000, 68, 215-223 3. Maggi L., Bruni R., Conte U. “High molecular weight polyethylene oxides (PEOs) as an alternative to HPMC in controlled release dosage forms”, Int. J. Pharm, 2000, 195, 229-238 4. Reza Selim Md., Quadir M.A., Haider S.S. “Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery”, J. Pharm. Pharmaceut. Sci, 2003, 6(2), 274-291 5. Yang L., Fassihi R. “Examination of drug solubility, polymer types, hydrodynamics and loading dose on drug release behavior from a triple-layer asymmetric configuration delivery system”, Int. J. Pharm, 1997, 155, 219-229 6. Wise, Donald L. Handbook of Pharmaceutical Controlled Release, New York Marcel Dekker, Inc., 2000, 155-179, 183-205, 255-267 7. www.colorcon.com 8. Klein S. Dissolution testing in the 21st century: design of a dissolution test equipment. Farmacia, 2005, 53, 3-11