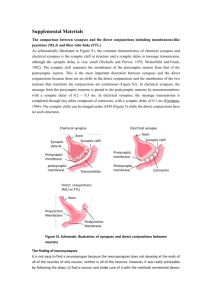
Supplementary Table 1: Supporting Morphological Results
advertisement

1 Supplementary Table 1: Supporting Morphological Results WTEM MUTEM WTLM MUTLM numIHCs 109 110 80 60 numafferent synapses 250 210 909 701 fractionribbon-containing synapses (%) lengthpostsynaptic density (nm) 42.4 0.4 91.3 12.4 746 ± 26 823 ± 57 - - (n = 42) (n = 20) 230 ± 9 - 255 - - 332 - - 200 - - - - 3.0 ± 0.2 1.9 ± 0.3 - - (n = 33) (n = 18) - - heightribbon/4Pi RIBEYE spot (nm) (n = 42) lengthribbon/4Pi RIBEYE spot (nm) 278 ± 18 (n=9) widthribbon/4Pi RIBEYE spot (nm) 55 ± 8 (n=9) numribbon-associated SV in 2D 14.2 ± 0.5 (n = 33) numdocked SV in 2D diameterSV (nm) distanceribbon-SV (nm) 30.0 ± 0.3 27.8 ± 0.3 (n = 206, cv = 15.2) (n = 202, cv = 13.7) 26.2 ± 0.6 - - - - 260 ± 4 (n = 201) 220 ± 20 (n = 195) sizesmall RIBEYE spots, 4Pi - (n = 15) (nm) sizebig RIBEYE spots, 4Pi (nm) (nm2) surface numribbon-associated SV 3D numdocked SV 3D RRPsynapse (vesicles/synapse) 763 ± 12 1273 ± 70 (n = 38) (n = 28) - ellipsoid:~3.82e5 - ~125 - sphere:~3.79e5 ellipsoid:~203 - ~16 - ellipsoid:~30 - 53-64 ribbon- ~58ribbon-contain.(1) - - ~2.38e5 - containing (10) ~14ribbon-deficient(11) (conversion factor: Cm/SV) (28 aF) (24 aF) RRPIHC 15-18 5 (fF) Table presents morphological findings obtained on WT and mutant IHCs of 8week-old mice. In the electron microscopy of 7 WT (WTEM) and 6 mutant (MUTEM) mice synapses (numsynapses) were identified as contacts of IHC (numIHCs) and dendrite displaying pre- and postsynaptic densities in ultrathin sections. One cochlea of each of the 7 WT and 6 mutant mice was processed. In our confocal analysis of 7 WT (WTLM) and 5 mutant (MUTLM) mice numIHCs 2 and numsynapses represent the total counts of CtBP2-positive IHC nuclei and GluR immunofluorescent spots, respectively, which we observed in animated 3D reconstructions of organs of Corti (see Supplementary Movies 1a and 1b). The number of RIBEYE-juxtaposed GluR spots was related to numsynapses to yield the fraction of ribbon containing synapses. RIBEYE-juxtaposed GluR spots were identified, when we found signals to be in contact by visual inspection. Counting revealed a strong reduction of ribbon-containing synapses in electron microscopy (along with abundance of floating ribbons and accumulation of cisterns) and light microscopy, allowing 100% correct genotype prediction. We captured many more synapses per investigated IHC and also obtained a much larger fraction of ribbon containing synapses in both WT and mutant IHCs in the confocal analysis. We suspect an overestimation of mutant ribbon-containing synapses due to insufficient lightmicroscopical separation of some close but not synaptically related ribbon and GluR signals in our confocal analysis (e.g. floating ribbons of Fig. 1c). On the other hand, we probably missed a significant number of anchored ribbons in electron microscopy mainly because, depending on its orientation with respect to the cutting axis, the ribbon may span only a single section and the series of thin sections was often not complete. Therefore, electron microscopy and confocal measurements provide lower and upper estimates of the fraction of ribbon-containing synapses. In order to relate our functional results to synapse morphology we semiquantitatively analysed 42 WT and 20 mutant synapses in single ultrathin electron microscopy sections and performed 4Pi microscopy on RIBEYE stained apical organs of Corti from WT and mutant mice (Fig. 1i-j). From electron microscopic observations we conclude that the average ribbon of mature mouse IHC takes an ellipsoid shape. Only ribbons with a perpendicular orientation with respect to a sharply delimited postsynaptic 3 density were analysed. The maximal extensions along the vertical and horizontal axes were taken as height and ‘width’ estimates. Due to random 2D orientation of ribbons in the sections ‘width’ estimates ranged between 25 and 354 nm (data not shown). For a rough approximation of ribbon surface from the electron microscopy data we averaged the lowest 20% of this distribution to yield an apparent width (“widthribbon“) and the highest 20% groups for an apparent length (“lengthribbon”). The 4Pi estimates of the three principal axes were obtained by approximating the major peak of the WT RIBEYE size distribution by a model that assumed an ellipsoid shape and random orientation of the ribbon with respect to the optical axis (Fig. 1k, Supplementary Methods). The number of ribbon-associated synaptic vesicles in 2D sections included all vesicles within 30 nm distance from the ribbon. Docked synaptic vesicles touched the presynaptic membrane and were dominated by ribbonassociated synaptic vesicles (~ 2/3) in WT synapses. The total number of docked synaptic vesicles in EM was roughly approximated based on the 2D count of 3 docked synaptic vesicles, assuming a hexagonal packing with a 55% density along the ribbons length plus 3 synaptic vesicles at each end of the ribbon. Although we cannot rule out that our fixation paradigm caused transmitter release and subsequent synaptic vesicles depletion, we do not favour this hypothesis, because different from strongly stimulated ribbons1,2 our WT IHC ribbons were densely populated with synaptic vesicles also at their base. In fact, our EM estimate of docked synaptic vesicles probably represents an upper estimate rather than an underestimate, since the 2D estimate of ribbon-associated synaptic vesicles lumped together slim and wide ribbon cross-sections. Synaptic vesicle diameters were measured from the lipid bilayer’s centres and synaptic vesicle capacitances calculated assuming a specific membrane capacitance of 10 fF/µm 2 (ref. 3). We did not 4 correct for shrinkage due chemical fixation. Because of shrinkage we may have underestimated the vesicle size and hence further overestimated the number of docked and ribbon-associated vesicles. For a rough approximation of the number of ribbon-associated synaptic vesicles we calculated the surface around the ribbon that is available for vesicle packing based on EM and 4Pi ribbon shape estimates, the average distance of the vesicle from the ribbon (distanceribbon-SV) and the outer synaptic vesicle radius (17.5 nm). We assumed hexagonal packing of synaptic vesicles (1039 nm 2 hexagons) within this surface at a density of 55%1,2. To estimate the number of ribbonassociated vesicles from the 4Pi data with the least assumptions we also calculated a spherical packing surface around the mean thickness of the small RIBEYE spots, which gave nearly identical results. The 4Pi estimate of docked synaptic vesicles number relied on the assumption that docked ribbon-associated synaptic vesicles made up ~ 10% of all ribbon-associated vesicles as described for frog saccular hair cells1,2. We multiplied this estimate by 1.5 to roughly account for outlying vesicles (see above). The RRP of synaptic vesicles per synapse (RRPsynapse ) was estimated from exocytic Cm of Fig. 4, converted into vesicles numbers and related to the average number of synapse per IHC (Results section). For mutant IHCs we considered the remaining ribbon-containing synapse to hold as many synaptic vesicles as the average wt synapse (58 synaptic vesicles). The other synaptic vesicles were attributed to the ~ 11 ribbon-deficient synapses. 1. 2. 3. Lenzi, D., Runyeon, J. W., Crum, J., Ellisman, M. H. & Roberts, W. M. Synaptic vesicle populations in saccular hair cells reconstructed by electron tomography. J. Neurosci. 19, 119-132 (1999). Lenzi, D., Crum, J., Ellisman, M. H. & Roberts, W. M. Depolarization redistributes synaptic membrane and creates a gradient of vesicles on the synaptic body at a ribbon synapse. Neuron 36, 649-59. (2002). Breckenridge, L. J. & Almers, W. Final steps in exocytosis observed in a cell with giant secretory granules. Proc Natl Acad Sci U S A 84, 1945-9 (1987). 5