revisiongsheet final ans
advertisement

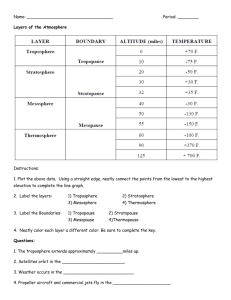
Port Said International Schools Revision sheet grade: 8 Better Education for Future Generations Science Department Name: ……………………………. --------------------------------------------------------------------------------------------------------------------------------- Unit one 1)Complete the following: 1-Mendeleev arranged the elements in ascending order according to their atomic weight 2-The advantages of Mendeleev period table are leaving spaces in his table and correcting the wrong atomic weights of some elements 3-Moseley arranged the elements in ascending order according to their atomic number 4-Elements are classified in the Modern periodic table according to their atomic numbers and the way of filling the energy sublevels with electrons 5- Modern periodic table consists of 7 horizontal periods and 16 vertical groups . 6-The atomic size is measured by bicometer 7- Water and ammonia are examples of polar compounds. 8-Each period in Modern periodic table starts with metal and ends with inert gas 9-The kind of elements which precede the inert gas in each period is halogens 10-Magnesium reacts with dilute acids and hydrogen gas evolved 11-Mg + 2HCl MgCl2 + H2 12- 2 Mg + O2 2MgO 13-MgO + H2O Mg(OH)2 14- CO2 + H2O H2CO3 15-Water molecule consists of 2 of hydrogen and 1 of oxygen. 16-The angle between the two single covalent bonds in water molecule is 104.5o 17-There are covalent bonds between water molecule. 18-When the water freezes its volume increases and its density decreases. 19-When acidified water decomposes by electricity H2 evolves above the cathode, while O2 evolves above the anode. 20-Metals oxides dissolve in water and form alkaline solution, while nonmetals oxides dissolve in water and form acidic solution ---------------------------------------------------------------------------------------------------------------2)Choose the correct answer: 1- The atomic number for element (Z) which lies in group (2A) and period 3 is ……… ( 12 - 6 - 20 - 18 ) 2- The atomic size of the elements of the same period…………………..by increasing their atomic number. ( increase - decrease - doesn't change ) 3-By increasing the atomic number, the electronegativity of elements of the same period……… ( increase - decrease - doesn't change ) 4-All of these elements metalloids except………………. ( silicon - sulpher - boron - arsenic ) 5- Nonmetallic property of the same group…............ by increasing the atomic number. ( increase - decrease - doesn't change ) 6- Metallic property of the same group…............by increasing the atomic number. ( increase - decrease - doesn't change ) 7-The physical state of iodine (I) is ……………………….. ( solid - liquid gas ) 8- On the electrolysis of a certain volume of acidified water if the volume of evolved gas at cathode is 8 cm3, so the volume of evolved gas at anode is…………cm3. ( 8 4 16 - 10 ) 9-There are ………………bonds between the water molecules. ( hydrogen - covalent - ionic - metallic) 10-Water has ………………effect on litmus paper. (basic - acidic neutral - alkaline) 11-Drinking water which contains high ratio of…………..leads to blindness. ( Mercury - Lead - Arsenic - Sodium ) 12-Each period in the modern periodic table ends with………………element. (metallic – inertgas – semimetallic – nonmetallic) 13-Alkaline earth metals are considered ……………….block groups. (s - p -d -f) 14- Metals oxides are ………… oxide. (acidic – basic – amphotoric – neutral) 3)Give reasons for: 1-Mendeleev left space (gaps) is his table. For predicting new elements which will discover 2-Some covalent compounds as sugar dissolve in water. Due to forming hydrogen bond 3-Water has high boiling point. Because of the presence of hydrogen bonds 4-Water has low density when it freezes. Because when the temperature decreases than 4 c the water molecules are collected by hydrogen bonds forming large sized hexagonal crystals with many spaces between them 5-Water used in fire extinguishing. Because it has high latent heat so it absorbs large amount of heat from the combustion media 6-Pure water has a neutral effect on both litmus paper. Because when water ionizes gives equal amount of positive hydrogen ions which is responsible for the acidic properties and negative hydroxide ions which is responsible for the basic properties 7-The atomic size of the elements of the same period decrease by increasing their atomic number Due to increasing the attraction force between the electrons and the nucleus. 8- The atomic size of the elements of the same group increase by increasing their atomic number Due to increasing number of energy levels 9-Alkali metals are kept under kerosene. To prevent its reaction with air because they are very active metals 10-Halogens are monovalent nonmetals Because they gain only one electron during the chemical reaction 11-Alkaline Earth metals are divalent elements Because they lose two electrons during the chemical reaction 12-Natural elements in group (17) don't exist individually. Due to their strong chemical activity 13-Silicon slides are used in manufacture of computers. Because they are semi-conductors which their conductivity of electricity depends on the temperature. 14- Liquefied nitrogen is used in preservation of the cornea of the eye. Because it has a low boiling point (-196). 15-Liquified sodium is used in transferring heat from inside the nuclear reactor to outside. Sodium is a good conductor of heat. 16- Elements of the same group have similar properties. Because they have the same number of electrons in the last energy levels. 4) What is meant by: 1. Chemical activity series. Arrangement of metallic elements in descending order according to their strong chemical activity. 2. Atom. The smallest building unit of the matter that can't exist in free state 3. Positive ion. Atom which lose one electron or more during chemical reaction. 4. Metalloids. Elements that have some physical properties of metals and others of nonmetals 5. Nonmetals. Elements which have more than 4 electrons in outermost energy level. 6. Electro negativity. It is the ability of the atom of covalent molecule to attract the electrons of the bond toward it. 7. Polar compounds. They are covalent compounds in which the difference electronegativity between elements forming their molecules is relatively high 8. Ionization process. It is the process of converting the molecules of some covalent compounds into ions 5) Locate the position of the following elements in the modern periodic table: 1. 4Be period 2 group 2A 2. 12Mg period 3 group 2A 3. 20Ca period 4 group 2A 6) Write the contribution of the following scientists : Mendeleev: first realistic table for classification of elements Bohr: discovered main energy levels Mosely: discovered that the periodicity of elements is related to their atomic number not their atomic weights Rutherford: discovered that the nucleus of atoms contain positively charged protons 7) Write the balanced chemical equations for the following reactions: 1. Burning of magnesium strip in oxygen 2Mg + O2 2MgO 2. Magnesium with hydrochloric acid Mg + 2HCl MgCl2 + H2 3. Burning coal in air C + O2 CO2 4. Carbon dioxide with water CO2 + H2O H2CO3 5. Dissolving magnesium oxide in water MgO + H2O Mg(OH)2 Unit two & three 1)Complete the following: 1-The measuring unit of the atmospheric pressure is millibar 2-The surface area at which the temperature is constant between troposphere and stratosphere is known as tropopause 3-The temperature decreases as going up in troposphere layer until it reaches -60 4- Ionosphere is surrounded by two magnetic belt known as Van Allen belts. 5-The satellites float around the Earth in an area known as exosphere where the atmosphere is inserted into outer space. 6-The air movement in troposphere is vertically while the air movement in stratosphere is horizontally 8- Hallons are from the pollutants of ozone layer which are used in fire extinguishers. 10-Methyle bromide gas is from the pollutants of ozone layer which is used as insecticide to preserve stored agricultural crops. 11-The most important greenhouse gases are CO2, CFCs and CH4 12-Examples of a complete body fossil are amber and mammoth fossil 13- Petrified wood & dinosaur's egg are examples of petrified fossils. 14- Studying the fossil record showed that the first vertebrate appeared was fish 15-Archaeopteryx represents the link between birds and reptiles 16-From factors causing extinction of species are over hunting and destroying natural habitats 2) Choose the correct answer: 1-The atmospheric pressure……………as we rise up above the sea level. (increases decreases - doesn't change ) 4- All the following are greenhouse gases except…………… (CO2 - O2 - N2O - CH4 ) 5-Ozone layer is measured by a unit called…………… ( km nm Dobson - mm3 ) 6-Most of the weather features occur in ………….layer (troposphere - thermosphere - mesosphere - stratosphere ) 7-……………..are used in extinguishers (methyl bromide gases - halons - nitrogen oxides - UVradiation) 8-The scientists have noticed there was erosion of ozone layer above………….. ( north pole - south pole - equator ) 9-When CFC compounds breaks down under the effect of UV radiation ……….atom liberates. (chlorine fluorine - carbon - oxygen ) 10-…………………is from the endangered animals because of global warming. ( Blue whale - seal - shark ) 11-The movement of air is vertical in …………layer. (troposphere - stratosphere - mesosphere - thermosphere ) 12-fossils are often found in ………………….rock. ( metamorphic - sedimentary - volcanic - igneous ) 13-……........fossils, they indicate that the environment where they lived was a hot and rainy tropical environment. ( Nummulites - Ferns - Coral ) 14-……........fossils, they indicate that the environment where they lived was clear, warm and shallow seas. ( Nummulites - Ferns - Coral ) 15- -……........fossils, they indicate that there was a sea floor in Gebel Mokattam. ( Nummulites - Ferns - Coral ) 16- Complete fossils of insects are found preserved in……………………… (ammonites - amber - igneous rock - metamorphic rock ) 17-………………. is a replica of the internal details of an ancient organism. (Mold – Cast – Trace – Petrified fossil) 18- ………………. is a replica of the external details of an ancient organism. (Mold – Cast – Trace – Petrified fossil) 3) Give reasons for: 1- Ionosphere is important for radio stations.. As it reflects radio waves that transmitted by communication centers and radio stations 2-The lower part of the stratosphere is suitable for flying airplanes. Because it doesn’t contain weather disturbance and the air movement is horizontally. 3-The phenomenon, ozone hole, increases in September each year. Because all pollutants assemble as black clouds and are pushed by wind towards south pole making ozone depletion. 4- Stop building concord airplanes. Because they produce nitrogen oxide which causes erosion of ozone layer. 5 Mesosphere layer is much vacuumed. Because it contains limited amount of hydrogen and helium 6- Bald eagle is from endangered species. Because it feeds on fishes which contain poisonous matter. 7- Hunting of Tasmanian cat by peasants. Because it preys on chickens and sheep. 8- Disappearance of papyrus plant from upper Nile. Due to drought of the swamp. 4)Write the word(s) that means each of the following statements: 1- The continuous increase of the average temperature of the air near the surface of the Earth. (Extinction) 2- The weight of air column of an atmosphere height above a unit area. (Atmospheric pressure). 3-One of the atmosphere components that its ratio increases in recent years to reach 0.038% (CO2 ) 4-An extinct bird has short legs and its wings are small. (Dodo bird ) 5- An extinct bird ,its female lays only one egg each spring and its extinction due to cutting the oak and beech trees ( passenger pigeon) 6- An extinct mammal, midway between horse and zebra. (Quagga) 7- An extinct mammal has a wolf's head, dog's tail, a pouch like kangaroo and striped skin like a tiger. (Tasmanian cat) 8-An endangered mammal , it inhabits Bamboo forests in northeast China. (Panda bear) 9- An endangered mammal, it overhunted for using its horn for medical purposes. (Rhino) 10- An endangered bird , its head covered with white feathers. (Bald eagle) 11- An endangered bird, it disappeared from Aswan after the building of the High Dam (Ibis bird) 12- An endangered plant, it grow in swamps of the Upper Nile, it was used to manufacture writing papers. (Papyrus plant) 5)Choose from column(B)what suit in column(A): A 1-Troposphere (f) 2-Mesosphere (e) 3-Stratosphere (d) 4-Mesopause (h) 5-Exosphere (b) 6-Ionosphere (g) B a- the hottest layer in the atmosphere envelope. b- it is the region in which the atmosphere envelope is inserted with the outer space. c- it is the region between mesosphere and stratosphere. d- it is the layer in which air moves horizontally. e- the coldest layer in the atmosphere envelope. f- the layer that all atmospheric phenomena happen in it. g- it has an important role in wireless communications . h- it is the region mesosphere and thermosphere. 6)Problem: 1-Calculate the height of a mountain if the temperature at its base 25oC and its top -14oC The temperature at the top of the mountain =temperature at its base –the decrease in temperature -14=25-the decrease in temperature The decrease in temperature =25 +14=39 So 39=height of mountain (km) x6.5Ċ So height of mountain =39÷6.5=6 km 2- Calculate the temperature at a point of height 2 km above the sea level if the temperature ate sea level is 24° C. The temperature at certain height=temperature at sea level-the decrease in temperature The temperature at certain height=24-(6.5x2km) The temperature at certain height=24-13 =11Ċ 7) What is meant by: 1- Atmospheric pressure? It is the weight of air column of an atmosphere height on unit area. 2- Aurora phenomenon? It is the phenomena that appears as brightly coloured light contains curtains seen from the both poles of earth. 3- Exosphere region? It is the region in which the atmospheric envelope is inserted with outer space. 4- Ionosphere? It is the layer that contains charged ions and it has an important role in wireless communications. 5- Global warming? It is the continuous increase in the average temperature of the earth's near surface air. 6- Greenhouse effect? It is the trapping of infrared radiations in the troposphere layer due to the increase of the ratio of green house gases which causes the increase of planet earth temperature. 8-Fossils Traces and remains of old living organisms which are preserved in sedimentary rocks. 9- Extinction Continuous decreasing in number of species members without compensation. 10- Simple ecosystem An ecosystem inhabited by small number of organisms and therefore is affected by the extinction of one of its organisms. 11- Complicated ecosystem An ecosystem inhabited by larg number of organisms and therefore isn't affected by the extinction of one of its organisms. 12- Natural protectorate Regions protected where hunting animals is restricted to increase the number of endangered species. 8) Mention the importance of : 1. 2. 3. 4. Aneroid: determination of possible day weather. Altimeter: determination the elevation of the navigation of the airplanes. Ozone layer: it protects us from the medium and far ultraviolet radiations Van Allen belts: it has an important role in scattering of harmful charged cosmic radiations 5. Satellites: used to transmit weather condition information and tv programs. 6. Halons: they are used in fire extinguishers . 7. Fern fossil: indicates that the environment where they lived was a hot and rainy tropical environment. 8. Coral fossil: indicates that the environment where they lived was clear,warm and shallow seas. 9. Nummulities fossil: indicate that there was a sea floor in Gebel Mokattam. 10. Radiolaria fossil: petroleum exploration. (2) 11. Hoffman voltmeter: electrolysis of water (1) 9) Look at the next figure then answer: 1- Label the next figure. (1) Oxygen (2) Hydrogen 10) Look at the next figure then answer: 1- Label the next figure. (1) Thermosphere (2) Mesosphere (3) Stratosphere (4) Troposphere