Botulinum toxin type A for headache prevention notification proforma
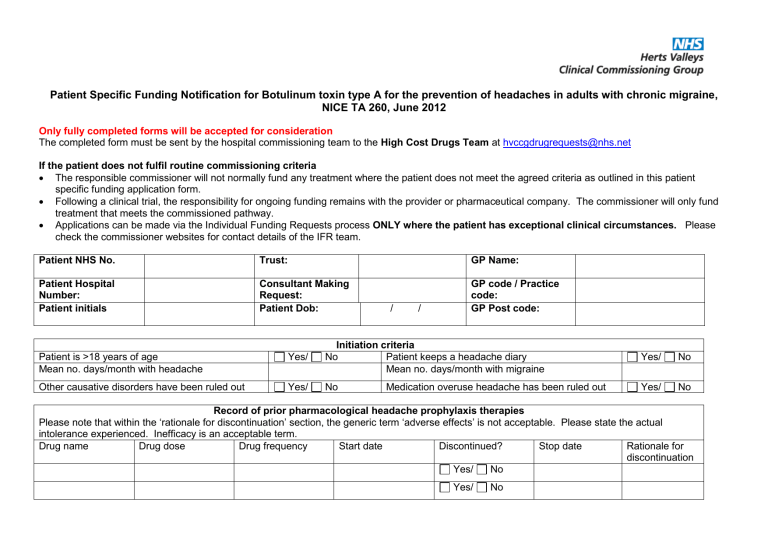
Patient Specific Funding Notification for Botulinum toxin type A for the prevention of headaches in adults with chronic migraine,
NICE TA 260, June 2012
Only fully completed forms will be accepted for consideration
The completed form must be sent by the hospital commissioning team to the High Cost Drugs Team at hvccgdrugrequests@nhs.net
If the patient does not fulfil routine commissioning criteria
The responsible commissioner will not normally fund any treatment where the patient does not meet the agreed criteria as outlined in this patient specific funding application form.
Following a clinical trial, the responsibility for ongoing funding remains with the provider or pharmaceutical company. The commissioner will only fund treatment that meets the commissioned pathway.
Applications can be made via the Individual Funding Requests process ONLY where the patient has exceptional clinical circumstances.
Please check the commissioner websites for contact details of the IFR team.
Patient NHS No.
GP Name:
Patient Hospital
Number:
Trust:
Consultant Making
Request:
GP code / Practice code:
Patient initials Patient Dob: / / GP Post code:
Patient is >18 years of age
Mean no. days/month with headache
Initiation criteria
Yes/ No Patient keeps a headache diary
Mean no. days/month with migraine
Yes/ No
Other causative disorders have been ruled out Yes/ No Medication overuse headache has been ruled out Yes/ No
Record of prior pharmacological headache prophylaxis therapies
Please note that within the ‘rationale for discontinuation’ section, the generic term ‘adverse effects’ is not acceptable. Please state the actual intolerance experienced. Inefficacy is an acceptable term.
Drug name Drug dose Drug frequency Start date Discontinued? Stop date
Yes/ No
Rationale for discontinuation
Yes/ No
Yes/ No
Retreatment criteria
For eligible patients, funding approvals will be given for a first and follow up injection (i.e. 2 injections) on each occasion.
First response assessment to be made following 2 nd injection.
Percentage reduction in headache days per month after initial 2 treatment cycles, measured over a period of at least a month
Date range for measurements
Number of headache days/month measured over 3 consecutive months Date range for measurements
Costing information
Drug cost to be charged for single injection
Is VAT payable on the drug cost?
Activity cost to be charged for administration (HRG code if known)
Yes/ No
Clinician’s Declaration
I confirm that I have discussed with the patient and that they understand and consent to their personal information being shared with commissioning organisations. I have also recorded this discussion in the patient’s notes.
I confirm the risks and benefits of treatment have been fully discussed with the patient and documented.
I confirm that funding approval is subject to initiation and follow up of treatment response being undertaken by a specialist who has attended a certified training course to administer botulinum toxin type A for headaches.
I acknowledge and adhere to the cost effective use of this treatment as advocated in NICE TA 260 and believe that within this Provider setting the above patient would be best managed using the treatment as requested above.
Name of consultant (or clinician delegated by consultant):
Signature (electronic signature):
Date: / /
If this patient is being jointly managed by a second consultant, please state name here:
Name :
Date :
Signature (or email confirmation) by Trust Chief Pharmacist (or nominated deputy)
Name:
Signature: Date: / /
Pharmacy and Medicines Optimisation Team
Herts Valleys Clinical Commissioning Group (HVCCG)
Botulinum toxin type A for the prevention of headaches in adults with chronic migraine
(NICE TA 260, June 2012)
NICE Recommendation:
1.1
Botulinum toxin type A is recommended as an option for the prophylaxis of headaches in adults with chronic migraine (defined as headaches on at least 15 days per month of which at least 8 days are with migraine):
that has not responded to at least three prior pharmacological prophylaxis therapies and
whose condition is appropriately managed for medication overuse.
1.2 Treatment with botulinum toxin type A that is recommended according to 1.1 should be stopped in people whose condition:
is not adequately responding to treatment (defined as less than a 30% reduction in headache days per month after two treatment cycles) or
has changed to episodic migraine (defined as fewer than 15 headache days per month) for three consecutive months.
HMMC Recommendation, following further clarification of NICE TA260 with specialists:
The treatment will be commissioned in line with a patient specific notification form.
The service providing the treatment is required to ensure that:
1. The patient’s headaches are not attributed to any other disorder.
2. The practitioner providing the injection has been trained by a certified trainer in giving the I.M injection in the appropriate areas of head and neck.
3. A process is in place to differentiate headache episodes from migraine. Patients should be required to keep a headache diary and given a leaflet outlining the sort of information they need to keep a record of on a daily basis. International Headache Society (HIS) criteria (Appendix 1, number 1a-e) for determining the days that qualify as migraine days are to be used to differentiate a migraine from a normal headache.
4. Patients referred to the service have had at least three prophylactic treatments from different drug groups (see below. For further information, consult the
HMMC botulinum Toxin type A in migraine recommendation document): a. Amitriptyline to at least 50 mg for at least 2 months b. Propranolol to at least 160 mg for at least 2 months c. Pizotifen to at least 1.5 mg for at least 2 months d. Topiramate to at least 100 mg for at least 2 months e. Sodium valproate to at least 1,000 mg for at least 2 months.
Where appropriate prophylactic treatments have not been tried, advice should be given to the referring GP and the patient. The injection should not be administered at this stage. Patients must be encouraged to keep a headache diary prior to re-referral to specialist.
5. Analgesia overuse headaches are assessed and managed appropriately, by the practitioner. In addition to headache and type, patients must be asked to note in the diary whether they took analgesics or triptans that day.
6. Patients should not be overusing analgesics for 2 months prior to consideration for botulinum toxin type A to ensure that analgesic overuse is not the cause of the migraine becoming chronic.
7. Stopping criteria outlined by NICE TA 260, and documented in retreatment criteria above are adhered to. Stopping criteria can be monitored by the service using the patient’s headache diary.
8. Providers must submit audit of treatment in line with these commissioning arrangements and allow the service and outcomes to be audited.
9. Where the provider is outside neurology services, commissioners must ensure that the service specification includes the guidance provided in the documents from the RCGP web-site and DH guidelines on Care Closer to Home. http://www.rcgp.org.uk/pdf/CIRC_PwSI%20Headache.pdf





