Chemistry
advertisement

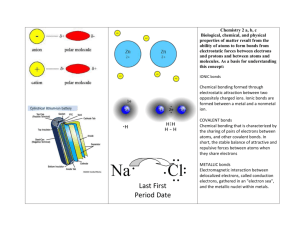
1. Chemistry Part 1: Basic Chemistry 2. Basic Chemistry Matter – States of matter Solid Liquid Gas 3. Elements Basic substances that make up matter Cannot be broken down by ordinary chemical means http://library.thinkquest.org/3616/chem/Periodic.ht m 4. Common Elements in the Human Body 5. Other Elements Lesser elements Iodine (I) Iron (Fe) Trace elements Often part of enzymes or required for enzyme activation Chromium (Cr), cobalt (Co), copper (Cu), fluorine (F), manganese (Mn), molybdenum (Mo), selenium (Se), silicon (Si), tin (Sn), venadium (V), and zinc (Zn) 6. Properties of Elements Each element has unique: Physical properties –detected with our senses (color, smell, freezing point) Chemical properties – pertain to the way atoms interact with one another (bonding behavior) 7. Atoms Almost identical building blocks for each element Atomic (chemical) symbol – one- or two-letter chemical shorthand for each element Consist of subatomic particles 8. Atomic Structure Nucleus Neutrons – have no charge and a mass of one atomic mass unit (amu) Protons – have a positive charge and a mass of 1 amu Electron Shells or Orbitals Electrons – have a negative charge and 1/2000 the mass of a proton (0 amu) 10. Identification of Elements Atomic number = number of protons Mass number = mass of protons and neutrons Isotope – atoms with same number of protons but a different number of neutrons _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ Atomic weight = average of the mass numbers of all isotopes Radioisotopes – atoms that undergo spontaneous decay called radioactivity 11. Identification of Isotopes http://images.encarta.msn.com/xrefmedia/aencmed /targets/illus/ilt/T046738A.gif 12. Molecules and Compounds Molecule – two or more atoms held together by chemical bonds Compound – two or more different kinds of atoms chemically bonded together 12. Chemical Bonds Electron shells, or energy levels, surround the nucleus of an atom Bonds are formed using the electrons in the outermost energy level Valence shell – outermost energy level containing chemically active electrons Octet rule – except for the first shell which is full with two electrons, atoms interact in a manner to have eight electrons in their valence shell 14. Chemically Inert Elements Outermost energy level fully occupied by electrons http://www.biologylessons.sdsu.edu/ta/classes/lab2 /argon.gif 15. Chemically Reactive Elements Outermost energy level not fully occupied by electrons http://www.biologylessons.sdsu.edu/ta/classes/lab2 /chnops.gif 16. Types of Chemical Bonds Ionic Covalent Hydrogen 17. Ionic Bonds Ions are charged atoms resulting from the gain or loss of electrons Anion -Atom that acquires a net - charge Has gained one or more electrons Cation -Atom that acquires a net + charge Has lost one or more electrons 18. Formation of an Ionic Bond Ionic compounds form crystals instead of individual molecules Crystals are large arrays of cations and anions joined by ionic bonds 19. Formation of an Ionic Bond http://www.ider.herts.ac.uk/school/courseware/materials/b onding.html _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ 20. Crystal Formation http://upload.wikimedia.org/wikipedia/commons/thumb/e/e b/Sodium_chloride_crystal.png/200pxSodium_chloride_crystal.png 21. Covalent Bonds Covalent bonds are formed by the sharing of two or more electrons Electron sharing produces molecules Covalent bonds may be single, double, or triple depending on the number of electron pairs that are shared 22. Single Covalent Bonds http://www.ider.herts.ac.uk/school/courseware/materials/b onding.html 23. Double Covalent Bonds http://faculty.clintoncc.suny.edu/faculty/Michael.Gregory/fil es/Bio%20100/Bio%20100%20Lectures/Biochemistry/bioche mi.htm 24. Nonpolar Covalent Bonds http://www.floridahyperbaricoxygen.com/assets/images/oxy gen_molecule1.gif 25. Polar Covalent Bonds http://faculty.clintoncc.suny.edu/faculty/Michael.Gregory/fil es/Bio%20101/Bio%20101%20Lectures/chemistry/img018.gif 26. Polar and Nonpolar Molecules [Figure B-5] Electrons shared equally between atoms produce nonpolar molecules Unequal sharing of electrons produces polar molecules 27. Comparison of Ionic, Polar Covalent, and Nonpolar Covalent Bonds Ionic Bond Polar Covalent Nonpolar Covalent Bond Bond Complete transfer Unequal sharing of Equal sharing of of electrons electrons electrons Separate ions Slight negative Charge balanced form charge at one end, among atoms slight positive charge at other + Water Carbon dioxide Na Cl 28. Hydrogen Bonds http://wwwcmls.llnl.gov/data/assets/images/science_and_techn ology/chemistry/water_str/mundy2.jpg Too weak to bind atoms together Polar molecules attracted to other polar molecules Responsible for surface tension in water Important as intramolecular bonds, giving the molecule a three-dimensional shape __________________________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ 29. Chemical Reactions Occur when chemical bonds are formed, rearranged, or broken Chemical equations contain: Number and type of reacting substances (reactants) and products produced Relative amounts of reactants and products 30. Examples of Chemical Reactions 31. Reversibility in Chemical Reactions All chemical reactions are theoretically reversible A + B AB AB A + B If neither a forward nor reverse reaction is dominant, chemical equilibrium is reached A + B AB 32. Factors Influencing Rate of Reactions Temperature – faster at higher temperatures Particle size – the smaller the particle the faster the chemical reaction Concentration – higher concentrations produce faster reactions Catalysts – increase the rate of a reaction without being chemically changed Enzymes – biological catalysts, usually proteins 33. Mixtures Mixtures – two or more components physically intermixed (not chemically bonded) Three types of mixtures exist: solutions, colloids, and suspensions Most matter in nature exists as mixtures 34. Solutions Solutions – homogeneous mixtures of components Solvent – substance present in greatest amount Solute – substance(s) present in smaller amounts May be gas, liquid, or solid 35. Electrolytes/Nonelectrolytes Ions in solution conduct electricity Cations and anions separate and are distributed equally throughout the solution Electrolytes – solutes that form ions and conduct electricity Nonelectrolytes – solutes that do not form conductive solutions _ 36. Concentration of Solutions Percent, or parts or solute per 100 parts of solvent Molarity, or moles per liter (M) A mole of an element or compound is equal to its atomic or molecular weight (sum of atomic weights) in grams _________________________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ One mole of any substance contains exactly the same number of solute particles (6.02 x 1023) 37. Colloids and Suspensions Colloids, or emulsions, are heterogeneous mixtures whose solutes do not settle out Suspensions are heterogeneous mixtures with visible solutes that tend to settle out 38. Mixtures vs. Compounds No chemical bonding takes place in mixtures Most mixtures can be separated by physical means Mixtures can be heterogeneous or homogeneous Chemical bonds occur between the components Compounds cannot be separated by physical means All compounds are homogeneous 40. Biochemistry Organic compounds Contain carbon (and hydrogen) Covalent bonds CO and CO2 are not organic Inorganic compounds Do not contain carbon Water, salts, and many acids and bases 41. Inorganic Compounds – Water http://www.chemistryland.com/CHM107/Water/Wa terTableSalt.jpg Inorganic Compounds - Salts 42. Ionic compounds - Salts Dissociate Contain cations (other than H+) and anions (other than OH–) Are electrolytes - conduct electrical currents 43. Inorganic Compounds - Acids and Bases Acids contain H + and anions Release H+ and are therefore proton donors HCl H+ + Cl – Bases contain OH – and cations Release OH– and are therefore proton acceptors NaOH Na+ + OH– 44. Acid-Base Concentration (pH) Acidic solutions have higher H+ concentration and therefore a lower pH Alkaline solutions have lower H+ concentration and therefore a higher pH Acidic: pH 0–6.99 Basic: pH 7.01–14 Neutral: pH 7.00 Neutral solutions have equal concentrations of H+ and OH– 45. Acid-Base Concentration (pH) __________________________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ http://staff.jccc.net/PDECELL/chemistry/phscale.gif 46. Neutralization Mixing acids and bases leads to displacement reactions that restore pH to neutral Acid + Base Water + Salt HCl + NaOH H2O + NaCl 47. Buffers Chemical systems that resist changes in the pH of body fluids If pH increases, buffers release H+ If pH decreases, buffer bind H+ Carbonic acid – bicarbonate system 48. Organic Compounds Molecules unique to living systems contain carbon and hence are organic compounds They include: Carbohydrates Lipids Proteins Nucleic Acids 49. Monomers/Polymers Monomer Single unit or building block Simple sugars, fatty acids, amino acids, nucleotides Polymers Many units Complex carbohydrates, lipids, proteins, nucleic acids 50. Carbohydrates Contain carbon, hydrogen, and oxygen (1:2:1) Supply cellular fuel Examples: Monosaccharides or simple sugars (3-7C) Pentose and hexose sugars are key 51. Carbohydrates Disaccharides or double sugars 52. Carbohydrates - Fig. 2.14 Polysaccharides or polymers of simple sugars Glycogen (animal), starch (plant), cellulose 53. Lipids Contain C, H, and O, but the proportion of oxygen in lipids is less than in carbohydrates 54-55. Neutral Fats (Triglycerides) Composed of three fatty acids bonded to a glycerol molecule 56. Phospholipids __________________________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ Modified triglycerides with two fatty acid and a phosphorus group groups 57. Steroids flat molecules with four interlocking hydrocarbon rings Eicosanoids – derived from 20-carbon fatty acid found in cell membranes 58. Prostaglandins Regulatory compounds derived from membrane proteins 59. Lipids Found in the Body Neutral fats – found in subcutaneous tissue and around organs Energy source, insulation, cushion Phospholipids – chief component of cell membranes Steroids – cholesterol, bile salts, vitamin D, sex hormones, and adrenal cortical hormones 60. Lipids Found in the Body Fat-soluble vitamins – vitamins A, E, & K Eicosanoids – prostaglandins, leukotrienes, and thromboxanes Lipoproteins – transport fatty acids and cholesterol in the bloodstream 61. Amino Acids Building blocks of protein Contain an amino group and a carboxyl group 62. Protein Composed of combinations of 20 types of amino acids bound together with peptide bonds 63-66. Structural Levels of Proteins Primary – amino acid sequence Secondary - Alpha helices/Beta pleated sheets Tertiary – superimposed folding of secondary structures Quaternary – polypeptide chains linked together in a specific manner 67. Protein Denaturation http://www.sp.uconn.edu/~bi107vc/images/mol/de naturation.gif Irreversibly denatured proteins cannot refold and are formed by extreme pH or temperature changes 68. Nucleic Acids Storage and use of genetic information DNA (nucleus) and RNA (cytoplasm) Nucleotide – Nitrogen containing base Adenine (A), guanine (G), cytosine (C), thymine (T), and uracil (U) __________________________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ Sugar Ribose or deoxyribose Phosphate group 69. Deoxyribonucleic Acid (DNA) http://www.scq.ubc.ca/wp-content/dna.gif Double-stranded helical molecule found in the nucleus of the cell Replicates itself before the cell divides, ensuring genetic continuity Provides instructions for protein synthesis 70-71. Ribonucleic Acid (RNA) http://tigger.uic.edu/classes/phys/phys461/phys450 /ANJUM04/RNA_sstrand.jpg Uses uracil instead of thymine Three varieties messenger RNA transfer RNA ribosomal RNA 72-73. Adenosine Triphosphate (ATP) [Figure B-18] __________________________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________ _______________________________