in vivo biohazard containment evaluation
advertisement
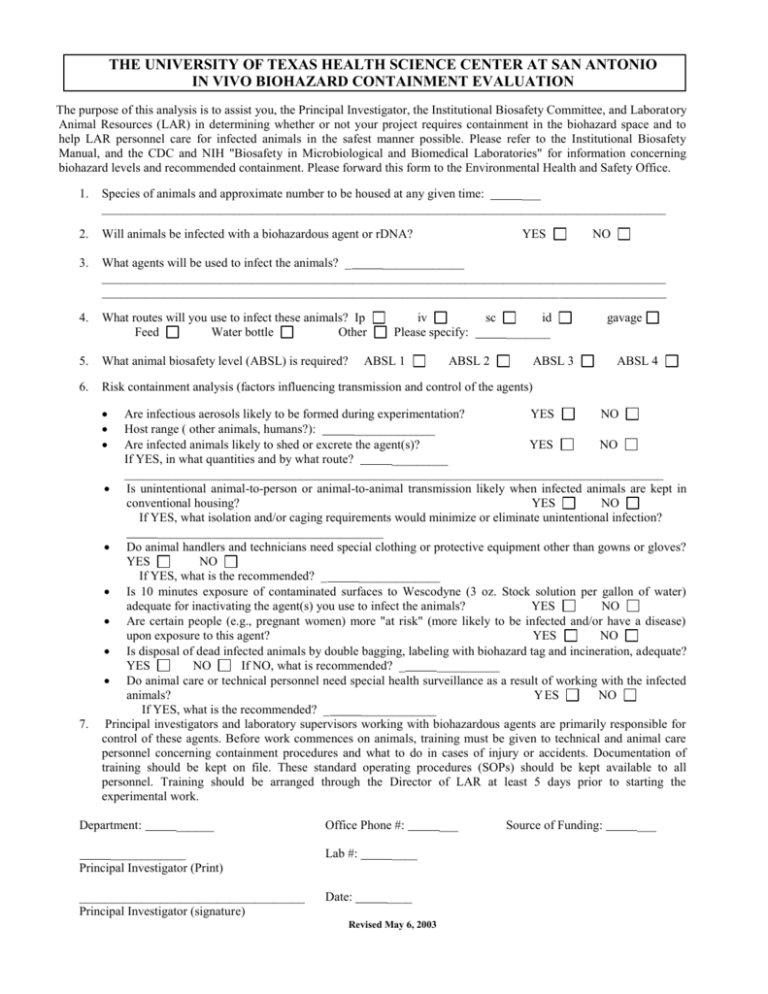
THE UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO IN VIVO BIOHAZARD CONTAINMENT EVALUATION The purpose of this analysis is to assist you, the Principal Investigator, the Institutional Biosafety Committee, and Laboratory Animal Resources (LAR) in determining whether or not your project requires containment in the biohazard space and to help LAR personnel care for infected animals in the safest manner possible. Please refer to the Institutional Biosafety Manual, and the CDC and NIH "Biosafety in Microbiological and Biomedical Laboratories" for information concerning biohazard levels and recommended containment. Please forward this form to the Environmental Health and Safety Office. 1. Species of animals and approximate number to be housed at any given time: ___ __________________________________________________________________________________________ 2. Will animals be infected with a biohazardous agent or rDNA? 3. What agents will be used to infect the animals? _ _____________ __________________________________________________________________________________________ __________________________________________________________________________________________ 4. What routes will you use to infect these animals? Ip Feed Water bottle Other 5. What animal biosafety level (ABSL) is required? 6. Risk containment analysis (factors influencing transmission and control of the agents) YES iv Please specify: ABSL 1 sc ABSL 2 NO id _______ ABSL 3 gavage ABSL 4 7. Are infectious aerosols likely to be formed during experimentation? YES NO Host range ( other animals, humans?): _____________ Are infected animals likely to shed or excrete the agent(s)? YES NO If YES, in what quantities and by what route? _________ ______________________________________________________________________________________ Is unintentional animal-to-person or animal-to-animal transmission likely when infected animals are kept in conventional housing? YES NO If YES, what isolation and/or caging requirements would minimize or eliminate unintentional infection? ____________________________________ Do animal handlers and technicians need special clothing or protective equipment other than gowns or gloves? YES NO If YES, what is the recommended? _ _____________ Is 10 minutes exposure of contaminated surfaces to Wescodyne (3 oz. Stock solution per gallon of water) adequate for inactivating the agent(s) you use to infect the animals? YES NO Are certain people (e.g., pregnant women) more "at risk" (more likely to be infected and/or have a disease) upon exposure to this agent? YES NO Is disposal of dead infected animals by double bagging, labeling with biohazard tag and incineration, adequate? YES NO If NO, what is recommended? _ __________ Do animal care or technical personnel need special health surveillance as a result of working with the infected animals? Y ES NO If YES, what is the recommended? _ ____________ Principal investigators and laboratory supervisors working with biohazardous agents are primarily responsible for control of these agents. Before work commences on animals, training must be given to technical and animal care personnel concerning containment procedures and what to do in cases of injury or accidents. Documentation of training should be kept on file. These standard operating procedures (SOPs) should be kept available to all personnel. Training should be arranged through the Director of LAR at least 5 days prior to starting the experimental work. Department: ______ Office Phone #: ____________ Principal Investigator (Print) Lab #: ____________________________________ Principal Investigator (signature) Date: ___ Source of Funding: ____ ____ Revised May 6, 2003 ___ ___