Grant Proposal Core C Appendix (FISH Initiative)
advertisement

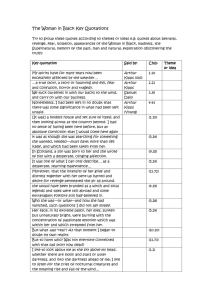
CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDICES CORE C DIRECTOR Laura Rassenti, PhD UCSD A. CRC Tissue Core Work Flow B. CRC Tissue Core Standard Operating Procedures (SOPs) C. Tissue Core Organizational Structure D. CRC Tissue Core Publications/References E. FISH Questionnaire F. FISH Data Collection Form G. Tissue Core Familial CLL Forms H. Tissue Core Informed Consent & HIPAA Forms Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDIX A. CRC Tissue Core Work Flow Tissue Core Treating P hysicia n Patient Si gns C onsent/Patie nt ID As signed Barcode Placed on P atient Fil e Data Core CRC CLL Patie t I T JK 0 00 1 Sample drawn & Buccal swabs coll ected, Bar Code Affixe d Wit h Patient ID Sample received Patient ID scanne d Sample ID created Sample sent Via Fed Ex ON delive ry to Tissue Core Plasma Collectio n Harve st PBMCs & freeze Patient Clini cal & Demographic data Collectio n & entry Confidential data separation Plasma ID & storage location l ogge d CRC CLL Plas ma T J K 0 0 0 1 - 0 5 2898 PBMC I D & storage location logg ed CRC CLL PBMC T J K 0 0 0 1 - 0 5 2 898 Tissue Core Serv ices Immunophenotyping(CLL & ZAP-70) IgVH subgroups & Mutational status Cyto/ FISH MRD,sCD20/CD52,HeavyWater(Cli nical) Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDIX B. CRC Tissue Core Standard Operating Procedures (SOPs) SOP # 1:Blood Processing Protocol The peripheral blood mononuclear cells (PBMCs) are isolated from the blood samples (and/or bone marrow samples) via density gradient centrifugation at 400 x g for 30 min. over a cushion of Ficoll-Paque PLUS™ (Pharmacia) (density 1.077 ± 0.001 g/m). This material has been tested for endotoxin and certified to have <0.12 EU/ml. The interface containing the lymphocytes are harvested and washed twice in RPMI-1640 (Irvine Scientific, CA). For this, the cells are suspended and then pelleted at 100 x g for 10 min. at 4o C. Cells are then suspended in ice-cold cryostorage media (10% DMSO, 90% FCS) at a concentration no greater than 108 cells per milliliter and aliquoted into 2ml cryostorage vials that have been labeled with a sample ID barcode. The vials are then frozen at a controlled rate in Controlled Rate Freezing Containers (Nalgene, Fisher Scientific, Tustin, CA.) The next day vials are placed into a liquid nitrogen storage system in the vapor phase and the vial locations logged with their sample ID in the Sample Storage database. DNA will be extracted from 5-10 x 106 cells, as QIAGEN, Santa Clarita, CA described The DNA is using the DNA Purification kit (QIAGEN, Santa Clarita, CA), (stored in 10-20 µg aliquots at –70o C in TE (10 mM Tris, pH. 7.4, 1 mM EDTA). Total RNA is extracted from 1 x 107 cells, using RNeasy kit (QIAGEN, Santa Clarita, CA), SOP # 2:Collection of Cheek Cells & DNA isolation 1) Remove the swab from the package, do not touch the foam tip. 2) Place the foam tip in the patient’s mouth. Firmly move the swab around the inside of both cheeks. Use both sides of the foam tip to soak up as much saliva as possible. 3) Place the patient name and date of collection on the FTA card. 4) Open the FTA classic card. Firmly press, do not rub, the foam tip on two of the circles until wet. Continue pressing until both circles are saturated with saliva. Repeat step 2 and continue wetting the remaining 2 circles with the foam tip. 4) Allow the FTA card to air-dry at room temperature for 30min. 5) Place the FTA card inside the white foil-lined envelope and seal the envelope. 6) Place the white foiled-lined envelope into the Fed Ex envelope and mail it to the CRC Tissue Core, alternatively, you can send these cards in the box with the blood samples The FTA coated paper is a matrix that protects the immobilized genomic DNA for long-term storage and PCR amplifications. When a biological sample (such as saliva) is spotted onto the FTA paper cells are lysed and the nucleic acids are immobilized within the matrix of the paper. The bound DNA can be purified by washing out the PCR inhibitors and adding add 200ul FTA Purification reagent. The DNA is then amplified directly from the paper by adding the PCR mix. SOP # 3: Collection of CLL cells at specific intervals during clinical protocols, A standard operating procedure will be used to obtain and process all samples. Each patient on protocol will have a 10 ml heparinized tube of venous blood removed just before administration of a CRC sponsored agent. The mononuclear cells will be isolated by isopycnic centrifugation, washed twice in cold RPMI 1640 medium and frozen in liquid nitrogen in the same medium supplemented with 20% autologous plasma and 10% DMSO at a cell density of about 5x10^6 per ml. Depending on the protocol supplemental blood samples will be taken hours, or days thereafter and processed in an identical fashion. Excess plasma will also be frozen. A complete blood count and differential will accompany each blood sample. The viable frozen CLL samples will be sent in dry ice for overnight delivery to the CRC Tissue Core and stored there, as described earlier. When the clinical trial is complete, the blood samples will be thawed, resuspended in media with autologous plasma and cultured. Thus each patient will have a before treatment culture, and several after treatment culture. The cells will be maintained in culture for up to four days, and viability will be Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. monitored by Flow Cytometry. Corresponding to the viability studies, extracts will be prepared from a proportion of the cells to monitor levels and phosphorylation states of specific molecules involved in signal transduction and apoptosis, according to the individual protocol. For each patient, at each time point, the subsequent culture data will reveal the number of days to produce 50% loss of viability of the recovered cells. All patient samples will be labeled according to the Tissue Core procedures with the patient ID, identification of the specific clinical trial, and time points of the treatment. All such data will be recorded in the CTMS and will be accessible to the CRC investigators upon request (Core A and Core D). SOP #4 : CLL Immunophenotyping Panel: The cells (5 x 105/100 µl) are washed and suspended in staining media (SM) which consists of 1x Hanks’ balanced salt solution (HBSS), 3% fetal calf serum (FCS), .01% NaN3, and 1 µg/ml propidium iodide (Calbiochem, La Jolla, CA), plus saturating amounts of specific mAb conjugated to fluorescein-isothiocyanate (FITC), phycoerythrin (PE), allophycocyanin (APC), or PerCP (PharMingen, San Diego, CA), allowing for four color immunofluorescence analyses. After 30 min at 4o C, cells are washed in SM and analyzed on a FACS-Calibur™ (Becton Dickinson, San Jose, CA). Dead cells are excluded from the analysis by the characteristic forward and side scatter profiles and the propidium iodide staining. The relative level of the antigen detected on the surface of the B cell is quantified as the mean fluorescence intensity ratio (MFIR). MFIR is the mean fluorescence intensity (MFI) of cells stained with a specific FITC-conjugated monoclonal antibody divided by the MFI of cells stained with a control IgG-FITC. We designate a CLL B-cell population as being positive for the expression of a specific antigen if its MFI is at least 1.25 times that of the leukemia cells stained with an isotype control, and when over 10% of the specific monoclonal antibody -stained cells show higher fluorescence than 99% of the control monoclonal antibodystained cells (Section D, Table 6). SOP # 5 : Immunophenotyping for ZAP-70: For ZAP-70 flow cytometry, the PBMC(5X10^5 cells) are stained for 20 min at 4°C with CD19- and CD3-specific mAbs conjugated with allophycocyanin (APC) and phycoerythrin (PE), respectively (PharMingen, San Diego, CA). The cells are washed twice and fixed with 4% paraformaldehyde in PBS and then permeabilized with Saponin in HBSS for 5 min at 4°C. The cells are washed and then stained with alexa-488-conjugated mAb specific for ZAP-70 (Clone 1E7.2) for 45 min at 4°C. Samples were washed and analyzed via flow cytometry using a FACS-Calibur® (Becton Dickinson, San Jose, CA) and Flow Jo 2.7.4 software (Tree Star, Inc., San Carlos, CA). We gate on lymphocytes using their forward-angle light-scatter (FSC) and side-angle light-scatter (SSC). Quadrants are set on gated cells such that 0.1% of the total lymphocytes were in the upper righthand quadrant. This gating is used on all the subsequent samples in the experiment. The expression of ZAP-70 is measured by calculating the percent of CD19+CD3-negative cells that is above this gating threshold. In each experiment we use control cells from healthy donors, CLL cells with high-levels of ZAP-70, and CLL cells with low-levels of ZAP-70. Fluorochromeconjugated, isotype control mAbs of irrelevant specificity were used in all experiments to monitor for non-specific staining. A positive of ZAP-70 expression is determined as being > 20% (Appendix D,#22). SOP # 6 : IgVH subgroup determination: Total RNA is isolated from 1X107 leukemic cells using RNeasy (QIAGEN,Santa Clarita, CA). The RNA is converted to cDNA using an oligo-dT primer and Superscript reverse transcriptase (Life technologies, Gaithersburg, MD). The Anchored PCR (aPCR) technique is based on the method initially described by Loh and colleagues. The dG tailed first strand cDNA is subjected to primary aPCR amplification in 100 µl of 10 mM Tris-HCL, 50 mM KCl, 1.5 mM MgCl2, 0.001% gelatin, 200 µM dNTPs (Pharmacia), and 2.5 µl Taq DNA polymerize (Boehringer Mannheim, Indianapolis). Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. The primers consist of specific Ig VH anti-sense oligonucleotide and anchor primers specific for the different Ig VH genes at a concentration of 1 µM. The amplification reaction consists of 20 cycles of 95o C x 30 s, 48o C x 30 s and 72o C x 1', followed by a final extension for 7 min. The product is purified using the QIAGEN PCR purification kit. A third of the purified product is used in a "nested PCR", using a 5' biotinylated antisense primer. Twenty cycles of PCR are carried out at an annealing temperature of 53o C and the products are purified using the QIAGEN PCR purification columns. This last PCR is performed to increase specificity and to attach biotin molecules at the 5' ends of the anti-sense strands for the ELISA. Oligonucleotide probes specific for the anti-sense strand of selected Ig VH genes, Ig VH gene sub-subgroups or Ig VH gene subgroups each are labeled with digoxigenin in a mixture containing 20 µl of 5 µM oligonucleotide, 200 mM potassium cacodylate, 25 mM Tris-HCL, 250 µg/ml BSA, 5 mM CaCl2, 50 µM digoxigenin-dideoxy-UTP (Boehringer Mannheim, IN) and 50 U terminal deoxynucleotidyltransferase. Subsequently, 150 µl of a 25 µM solution of each digoxigenin labeled sense-strand oligonucleotide detection probe in 6X SSC, 0.1% N-lauroylsarcosine are placed in separate wells containing plate-bound biotinylated anti-sense strand PCR products. The plates were heated at 65o C for 20 min and incubated for 90 min at 42o C. After incubation, the wells are washed 3 times with 6X SSC, 0.1% Nlauroylsarcosine, and once with blocking buffer (0.5% Genius blocking reagent- Boehringer Mannheim). This allows for specific hybridization of a sense strand oligonucleotide probe to the plate bound antisense-strand. Subsequently, the plates are incubated with peroxidase-conjugated anti-digoxigenin antibodies in blocking buffer for 30 min. The wells are washed once with blocking buffer, twice with Tris HCl buffer, and then incubated with 100 µl of tetramethylbenzidine peroxidase substrate (Kirkegaard and Perry Laboratories, Gaithersburg, MD). RNA The reaction is stopped with 1M phosphoric acid and after 30 min., OD values are recorded at 450 nm using an ELISA Ig cDNA microplate reader (Molecular Devices, Menlo Park CA). Anchor PCR The frequency of use of each Ig V gene is calculated by StrepAv well Biotin dividing the concentration of each V gene product by the total concentration of Ig V genes. The solid phase is prepared for the ELISA by adding 200 µl of a 0.1% solution of gluteraldehyde (Polysciences, Warrington, Pa.) in phosphate-buffered StrepAv well saline (PBS) to the individual wells of a polychlorovinyl ELISA microtiter plate (Falcon, Oxnard, CA). After 15-min Digoxigenin-labeled StrepAv well Ig V H probes incubation, the plate is washed with PBS. The wells are then incubated with 200 µl of 0.1 mg/ml Streptavidin (Sigma, St.Louis, MO) in PBS at 37 C for 2 hours. The wells again are washed with PBS. Unbound Streptavidin is Anti-digoxigenin removed by further adding 0.1% Triton X-100 (Sigma, peroxidase-conj.Ab St.Louis, MO) to each well and an incubation of 30 min. StrepAv well Subsequently each well is filled with a 1% solution of bovine serum albumin (BSA) (RIA grade, Sigma, St. Louis, StrepAv well MO) in PBS. The plates are allowed to incubate for 2 h at ELISA room temperature before rinsing with PBS. The plates can then be used immediately for the ELISA-PCR or stored at 4o C in PBS for up to one week. Each biotin-aPCR product is suspended in 100 µl of 6 x SSC, 0.1% Tween-20, and distributed among Streptavidin-coated wells and incubated for 1 hr at room temperature. The antisense strand becomes tethered to the Streptavidin-coated wells via the biotin-Streptavidin interaction, this forming an almost irreversible bond. Plates then are washed with 0.1 N NaOH, this denaturing the double stranded PCR product and releasing the sense strand from the plate-bound anti-sense strand. This strand may be allowed to hybridize with digoxigenin-labeled sense-strand oligonucleotides that subsequently may be detected with peroxidase-labeled anti-digoxigenin Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. mAbs. Standard curves are generated using known concentrations of cloned Ig V genes representative of each Ig VH gene, Ig VH gene sub-subgroup or Ig VH gene subgroup selected for analyses. Furthermore, control plasmids containing inserts representative of each of the antisense sequences targeted in our analyses will be amplified for generation of another standard curve using the above techniques. Such plasmids allows us to generate amplified-biotinylated products containing each of the probed sequences at equal stoichiometry, allowing us to quality monitor the hybridization signal and to discern hybridization cross-reactivity of a given oligonucleotide probe for closely related anti-sense sequences. SOP # 7 : IgVH mutational status determination & MRD assessment: The VH gene expressed by the CLL B cells is originally determined by the RT-PCR-ELISA technique. The cDNA is amplified via PCR using a mixture of sense-strand oligonucleotide primers specific for the leader sequence of the expressed IgVH and an anti-sense oligonucleotide primer specific for Cµ. The PCR products are size selected by electrophoresis in 2% low-melt agarose. The products are excised and purified (MiniElute PCR Purification Kit, Quiagen, Valencia, CA). An upstream Cµ oligonucleotide is used for priming cDNA for fluorescence-dideoxy-chain-termination synthesis. The fragments were evaluated using an Applied-Biosystems 377 automated-nucleicacid-sequence analyzer (Foster City, CA). Nucleotide sequences are analyzed using DNASTAR (Madison, WI) and compared to sequences in V-BASE and GenBank databases. The percent homology to the closest germline IgVH is calculated from the number of nucleotide differences between the 5’-end of FR1 and the 3’-end of FR3. Sequences with <98% homology with the corresponding germline Ig VH sequence are considered mutated. The particular IgVH gene expressed, the percent homology of this gene to the germline equivalent, the D region, and the JH region are all recorded into the CRC relational database for retrieval by the CRC investigators upon request. Since the Tissue Core already has determined the sequence of the expressed IgVH gene of all patients samples when first enrolled in the CRC, we will perform direct sequencing with leader or consensus VH and CH primers from the banked cDNA to determine the MRD status of specific CLL samples from CRC Clinical Trials as requested by CRC investigators (as described in Tissue Core reference #61) SOP # 8: Sandwich ELISA for detecting soluble CD20/CD52 in patient plasma : For sCD20 ELISA 96-well polystyrene microplates (Maxisorb) are coated with monoclonal rabbit anti-goat IgG, H+L (Jackson ImmunoResearch Labs) diluted to 1g/mL with 0.05M sodium carbonate buffer, pH 9.5 (100L/well) and incubation overnight at 4C. The wells are then washed 5-6 times with 150L PBS-Tween 20-0.1% and blocked with 250L of 2% BSA- PBS-Tween 200.1%; 3 hours at 37C, followed by washing 5-6 times with PBS-Tween 20-0.1%. Next, the wells are coated with polyclonal goat anti-CD20 IgG (Santa Cruz Biotechnology); (100L/well) at a concentration of 1g/mL diluted with 2% BSA- PBS-Tween 20-0.1%; and incubation overnight at 4C. The wells are the washed 5-6 times with PBS-Tween 20-0.1%. Patient plasma (100L) and standards are added to the wells and incubated at RT for 3 hours with shaking. Purified CD20 used for the standards was prepared from BJAB cells and used at concentrations of 1500, 750, 375, 187.5, 93.4, 46.9, and 23.4g/mL. Following this incubation step, the wells are again washed 5-6 times with PBS-Tween 20-0.1%. HRP-conjugated rituximab diluted in 2% BSA- PBS-Tween 200.1% is the added to each well (100L) followed by 3 hr incubation at RT with shaking. The wells are again washed 5-6 times with PBS-Tween 20-0.1%. Peroxide substrate (100L/well) is added and incubated for 20 minutes at RT with shaking. The reaction is stopped by adding 50L of 1M OPhosphoric acid to each well and absorbance at 450nm is measured. CD20 concentrations are determined for the samples using the standard curve generated. For sCD52 ELISA 96-well polystyrene microplates are coated with CAMPATH-1M antibody. Plates are washed six times with PBS containing 0.01% Tween-20, blocked with 2% BSA in PBS Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. containing 0.01% Tween-20 for 3 hrs at 37°C and then washed in PBS containing 0.01% Tween20. Patient plasma (100L) for testing and standards are added to wells and incubated for 3 hrs at RT, then washed eight times with PBS containing 0.01% Tween-20. sCD52 is detected using the HRP-conjugated humanized anti-CD52 (alemtuzumab) diluted 1:400 in 2% BSA and 0.01% Tween-20. The wells are washed six times with PBS containing 0.01% Tween-20. Substrate (100L) is added to develop the color, and the plates were incubated for 15 to 30 minutes with constant shaking. The reaction was then stopped by adding 50 uL of 1M O-Phosphoric acid and plates are analyzed for sCD52 by measuring absorbance at 450nm. Serial dilutions of purified CD52 from cells collected from a patient with CLL are used to generate a standard curve. To to measure alemtuzumab levels, affinity-purified rabbit anti-rat IgG (whole molecule) absorbed with human IgG to prevent binding to human IgG (1ug/100uL in 0.05 M carbonate buffer, pH 9.4) will be added to 96-well polystyrene microplates and incubated overnight at 4°C. Plates are washed five times with PBS-Tween 20 (PBS containing 0.01% Tween 20; Fisher Scientific International) and blocked using 200uL BSA (2%) in PBS-Tween 20 for 3 hrs at 37°C. Plates are then washed six times with PBS-Tween 20 and 100ul of patient sample will be added to appropriate wells. Plates are incubated for 2 h at RT with slow shaking, after which they are washed at least 6 times with PBS-Tween 20 and incubated with 100ul of HRP-conjugated affinitypurified rabbit anti-human-Fc in PBS-Tween 20 containing 15% fetal calf serum for 2 hrs at RT with constant shaking. A further eight washes with PBS-Tween 20 are done, then 100ul of substrate (3,3'-5,5'-tetramethylbenzidine, Dako, Carpinteria, CA) will be added to each well. The reaction is be stopped with 50 uL of 1M O-Phosphoric acid and absorbance determined at 450nm, and a log reading of samples against control is calculated. Samples are tested in BSA solution or plasma from normal individuals. SOP # 9: Measurement of leukemic B cell turnover rates The method involves a non-radioactive compound (“heavy water”; D20) the risks to patients are minimal. Heavy water tastes, feels and smells exactly like “regular” water and has no known harmful effects at the doses that will be given here. D20 also occurs naturally, and indeed a minor component of the water we all ingest daily contains D20. The only known side effect of D20 ingestion is vertigo that is caused when the compound is administered too rapidly and equilibration within the semi-circular canals of the ear is tardy. This side effect does not occur with the administration schedule that we have outlined. In the unlikely event that dizziness occurs, this subsides spontaneously within three hours. By ingesting a small amount of D20, newly produced DNA (deoxyribonucleic acid) will be labeled in cells that are being made in the bone marrow. After determining body weight, patients will be asked to drink 90 ml twice a day for five days and then 60 ml every day for the following 11 weeks of the study, to maintain a constant amount of D20 in their bodies. If patients miss a day’s dose, they will take it as soon as they remember. However, they will not take the missed dose if it is time for the next dose. Instead, they will skip the missed dose, and then resume the usual dosing schedule. The B-CLL cell kinetics in the blood as measured by entry into and exit from the peripheral pool can be made with reasonable accuracy as long as absolute lymphocyte counts are available at the time of each blood draw and there is no major change in the size of the solid lymphoid compartment. In addition, the incorporation of 2H into PMNLs will serve as an indication of the maximum incorporation that can be achieved which will also permit more accurate calculations. Taken together these measurements will allow us to calculate a growth rate and a half-life in the blood for the B-CLL cells on an individual patient. The exact duration of time during which the patient will ingest the D20 will be determined by the results of blood studies performed during the ingestion period. This heavy water will be provided in sterile aliquots that the patient will ingest each morning. Patients will be asked to return once a week for the first month, and then once every 4 weeks thereafter to measure body weight and to donate 30 ml of blood for analysis. If the results of the study suggest that more D20 is needed to label the leukemia cells to evaluate Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. turnover, patients will be requested to ingest additional 60 ml portions daily, as needed. Patient participation will continue for a total of 4 months after stopping the ingestion of the D 20. Therefore, the likely duration of participation in the study will be 5-6 months, depending on how long the patient will need to ingest D20 to effect adequate labeling. This period will be determined based on the mass spectroscopic studies of the leukemic cells isolated from the blood obtained after the first 4-week period. Subjects will also undergo routine clinical blood tests and examination by their physician to assure safety and monitor any unexpected side effects. These evaluations will occur according to their usual care or treatment regimens. The preliminary studies mentioned above involved a 12-week period of 2H2O intake, followed by an eighteen to twenty four week follow-up. Since an essential component of this method is the need to analyze 2H-incopration into the DNA of a specific, purified population of cells, isolation of leukemic B cells is essential. ~0.5 – 1.0 x 106 CD5+CD19+ cells are currently used for mass spectrometry measurements. The current approach for attaining these cells involves: [1] removal of CD3+ T cells from PBMC by positive selection using anti-CD3 microbeads (Miltenyi Biotech., Auburn, CA), [2] positive selection of CD5+ B cells by incubating the CD3- fraction cells with anti-CD5 mAb conjugated with PE (Beckton Dickinson Immunostaining Systems; San Jose. CA), and [3] final isolation of CD5+ B cells with anti-PE mAb linked to beads (Miltenyi Biotec). These CD5+ fractions routinely contain 95% B cells. SOP # 10: Conventional Cytogenetics Testing (performed retrospective on selected patient samples) This testing will be performed restospectively only on selected cases that are of interest and that have shown some unusual phenotypic and/or genetic abnormalities. Cytogenetic analyses typically require three cell cultures that are initiated in 10 ml RPMI 1640 with 15% FBS and 0.7 ml of peripheral blood or bone marrow in a T-25 culture flask. The first culture (cell culture) is incubated for 24 hr at 37 C before harvesting. The second culture (synchronized culture) is incubated at 37o C for 6 hrs, then 0.7 ml of 10-7 M methotrexate is added and incubated for 17 hrs. Subsequently 0.07 ml of 10-5 M thymidine is added and incubated at 37o C for 5 hrs and the cells are harvested. The third culture (mitogen-stimulated culture) is generated by adding 0.1 ml of a 10 mg/ml solution of lipopolysaccharide solution for peripheral blood cultures and 0.5 ml of a 5mg/ml solution of phorbol ester and 0.5 ml of a 0.5 mg/ml solution of pokeweed mitogen for bone marrow cultures. After 72 hrs these cultures are harvested. Arresting the mitotic cells with a spindle-fiber inhibitor performs the mitotic harvest and metaphase separation and the metaphase cells are collected for cytogenetic analysis. Cultures are harvested after a 25-min. exposure to 10 mg/ml Colcemid. The cultures are then transferred to a 15-ml conical tube and centrifuged for 5 min at 1000rpms. The supernatant is aspirated and the pellet is suspended in 8 ml of a 0.075-M potassium chloride hypotonic solution and incubated for 20 min at room temperature. Two ml of Carnoys fixative (1:4 acetic acid:methanol) are added and the mixture is spun for 5 min at 1000 rpm. The supernatant is aspirated and 8 ml of fixative are added, followed by a 20-min incubation at room temperature. This is repeated with 3 washes of fixative. The slides are prepared and analyzed by routine GTG banding. Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDIX C . Tissue Core Organizational Structure QuickTime™ and a TIFF (LZW) decompressor are needed to see this picture. Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDIX D. CRC Tissue Core Publications/references CRC Publications that were made possible from samples distributed by the CRC Tissue Core via the TCMS that resulted from CRC Hypothesis Driven Studies. (Clinical & demographic data was used via the CTMS) (Core B & Core A Interactions) (Total n=87) Project 1 (Croce) (n=7) 1) Hallas, C., Pekarsky, Y., Itoyama, T., Varnum, J., Bichi, R., Rothstein, J.L. and Croce, C.M.: Genomic analysis of human and mouse TCL1 loci reveals a complex of tightly clustered genes. Proc. Natl. Acad. Sci., USA, 96: 14418-14423, 1999. 2) Bullrich, F.,Rasio, D., Kitada, S., Starostik, P., Kipps, T., Keating, M.,Albitar, M.,Reed, J.C. and Croce,C.M. ATM mutations in B-cell chronic lymphocytic leukemia. Cancer Res., 59: 24-27, 1999. 3) Bullrich, F., Fujii, H., Calin, G., Mabuchi, H., Negrini, M., Pekarsky, Y., Rassenti, L., Alder, H., Reed, J.C., Keating, M.J., Kipps, T.J. and Croce, C.M.: Characterization of the 13q14 tumor supressor locus in CLL. Identification of ALT1, an alternative splice variant of the LEU2 gene. Cancer Res., 61: 6640-6648. 2001. 4) Calin GA, Trapasso F, Shimizu M, Dumitru CD, Yendamuri S, Godwin AK,Ferrancin M, Bernardi G, Chatterjee D, Baldassarre G, Rattan S, Alder H,Mabuchi H, Shiraishi T, Hansen LL, Herlea V, Mauro F, Dighiero G, MovsasB, Rassenti L, Kipps T, Baffa R, Fusco A, Mori M, Russo G, Liu CG,Neuberg D, Bullrich F, Negrini M, Croce CM. A germline stop mutation inARLTS1, a new member of the ADP-ribosylation factor family, isassociated with familial cancers and reduced in vivo apoptosis. NEJM 2005 21;352(16):1667-76. 5) Calin, G.A., Dumitru, C.D., Shimizu, M., Bichi, R., Zupo, S., Noch, E., Alder, H., Rattan, S., Keating, M., Rai, K., Rassenti, L., Kipps, T., Negrini, M., Bullrich, F., and Croce, C.M.: Frequent deletions and down regulation of micro RNA genes miR15 and miR16 at 13q14 in chronic lymphocytic leukemia. Proc. Natl. Acad. Sci., USA, 99: 15524-15529, 2002. 6) Calin, G.A., Liu, C.-G., Sevignani, C., Ferracin, M., Felli, N., Dumitru, C.D., Shimizu, M., Cimmino, A., Zupo, S., Dono, M., Del’Aquila, M.L., Rassenti, L., Kipps, T.J., Bullrich, F., Negrini, M., and Croce, C.M.: MicroRNA profiling reveals distinct signatures in B-cell chronic lymphocytic leukemia. PNAS USA 101:11755-11760. 7) Calin, A.C. et al (2005) Familial Cancer Associated with a Polymorphism in ARLTS1. NEJM:352:16: 1667-1676. Project 2 (Carson) (n=7) 8) Hua XH, Genini D, Gussio R, Tawatao R, Shih H, Kipps TJ, Carson DA, Leoni LM. Biochemical genetic analysis of indanocine resistance in human leukemia. Cancer Res. 2001 61:7248-7254 9) Wu CCN, Castro JE, Motta M, Cottam HB, Kyburz D, Kipps TJ, Corr M, Carson DA. Selection of oligonucleotide aptamers with enhanced uptake and activation of human leukemia B cells. In press in Human Gene Therapy 2003 14(9):849-60. 10)Desheng Lu, Yandong Zhao, Rommel Tawatao, Michael D. Rosenbach, Gilbert Weidinger, Randall T. Moon, Lorenzo M. Leoni, Maripat Corr, Dennis A. Carson Wnt Signaling in Chronic Lymphocytic Leukemia. ASH Abstract 2003 11)Desheng Lu, Yandong Zhao, Rommel Tawatao, Michael D. Rosenbach, Gilbert Weidinger, Randall T. Moon, Lorenzo M. Leoni, Maripat Corr, Dennis A. Carson Activationh of the Wnt signaling pathway in Chronic lymphocytic leukemia. PNAS 2004 101(9):3118-23. 12)Hedvat, M, Jain A, Carson DA, Leoni LM, Huang G, Holden S, Lu D, Corr M, Fox W, and Agus DB Inhibition of HER-kinase activation prevents ERK-mediated degradation of PPARgamma. Cancer Cell 5:565-574, 2004 Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. 13)Kolluri SK, Corr M, James SY, Bernasconi M, Lu D, Liu W, Cottam HB, Leoni LM, Carson DA, Zhang XK The R-enantiomer of the nonsteroidal antiinflammatory drug etodolac binds retinoid X receptor and induces tumor-selective apoptosis. PNAS USA 102: 2525-2530, 2005 14)Barchechath SD, Tawatao RI, Corr M, Carson DA, Cottam HB Quinolinium salt as a potent inhibitor of lymphocyte apoptosis Bioorg Med Chem Lett 15: 1785-1788, 2005 Project 2 (Reed) (n=10) 15)Pedersen IM, Samuel T., Scott F. L., Salvesen G. S., Honda T., Gribble G.W., Suh N., Sporn M.B., Kipps T.J. and. Reed J.C, The triterpenoid CDDO-Imidazolide potently induces caspase activation and apoptosis of CLL B-cells, submitted 16)Schimmer AD., Walsh K., Pinilla C., Bonneau M., Wang Z., Krajewska M., Bonneau M., Pedersen IM., Kitada S., Scott FL., Bailly-Maitre B., Glinsky G., Scudiero D., Sausville E., Salvesen G., Nefzi A., Ostresh JM., Houghten RA., and Reed JC., Small-molecule antagonists of apoptosissuppressor XIAP exhibit broad anti-tumor activity, Cancer Cell, 2004, 5:25-35. 17)Schimmer AD., Pedersen IM., Minden MD., Reed JC., Bcl-2 and Apoptosis in Chronic Lymphocytic Leukemia, Curr. Treat. Options Oncol., 2003, 4:211-8. 18)Schimmer A, Pedersen IM , Kitada S, Eksioglu-Demiralp E, Minden MD, Pinto R, Mah K, Andreeff M, Kim, Youngsoo; Suh, Won Suk and Reed JC. Functional blocks in Caspase activation pathways are common in leukemia and predict patient-response to induction chemotherapy, Cancer Research, 2003, 63:1242-8 19)Pedersen IM, Kitada S, Leoni LM, Zapata JM, Karres J, Tsukada N, Kipps TJ, Choi YS, Bennet F Reed JC. Protection of CLL B-cells by a follicular dendritic cell line is dependent on induction of Mcl-1. Blood, 2002, 100:1795-801. 20)Pedersen IM, Kitada S, Schimmer A, Kim Y, Zapata JM, Charboneau L, Rassenti L, Andreeff M, Bennett F, Sporn MB, Liotta LD, Kipps TJ, Reed JC. The triterpenoid CDDO induces apoptosis in refractory CLL B cells. Blood 2002 100:2965-2972 21)Irene M. Pedersen,Juan Zapata,Temesgen Samuel, F. Scott, M. Sporn, T. J. Kipps, Guy Salvesen, John C. Reed. The Triterpenoid CDDO-Imidazolide Induces Apoptosis of CLL B-Cells, through a Bcl-2-Independent Mechanism and Synergizes with Fludarabine ASH Abstract 2003 22)Shinichi Kitada, Barbara Becattini, Marilisa Leone, Edward Monosov, Sharon Chandler, Thomas J. Kipps, John C. Reed, Maurizio Pellecchia. Rational Design and Real Time In-Cell Detection of the Pro-Apoptotic Activity of a Novel Compound Targeting Bcl-2/Bcl-XL. Acknowledgement: Special thanks to Dr. Rassenti, Ph.D., CRC Tissue Core Director, for providing CLL samples. ASH Abstract 2003 23)Massimo Mangiola, Shinichi Kitada, Irene M. Pedersen, Clemencia Pinilla, Thomas J. Kipps, John C. Reed. XIAP Antagonist-Induced Cell Death in B-Cell Isolated from Patients with Chronic Lymphocytic Leukemia and in NHL B-Cell Lines. Acknowledgement: Special Thanks to Dr. Rassenti, Ph.D., CRC Tissue Core Director, for Providing CLL Samples. ASH Abstract 2003. 24)Becattini B, Kitada S, Leone M, Monosov E, Chandler S , Zhai D, Kipps TJ, Reed JC, Pellecchia M: Rational design and real time in-cell detection of the pro-apoptotic activity of a novel compound targeting Bcl-XL. Chemistry & Biology Vol 11: 389-396, March 2004 Project 3 (Kipps) (n=15) 25) Widhopf, G. Rassenti, LZ, toy, TL, Gribben,JG, Wierda,WG,Kipps, TJ.(2004) Chronic Lymphocytic leukemia B cells of over one percent of patients express virtually identical immunoglobulins. Blood:104,2499-2504 26) Rassenti, LZ, Huynh,L, Toy TL, Chen,L ,Keating,MJ, Gribben,JG, Neuberg,DS, Flinn,IW, Rai, KR, Byrd,JC, Kay, NE, Greaves,A, Weiss,A, Kipps TJ, (2004) ZAP-70 is a stronger predictor of early disease progression than immunoglobulin mutational status in chronic lymphocytic leukemia. NEJM 351;9;893-901 27)LZ Rassenti, TL Toy, L Huynh, A Greaves, JG Gribben, MJ Keating, KR Rai, IW Flinn, JC Byrd, NE Kay, LR Goldin, NE Caporaso, TJ Kipps. The CLL Research Consortium. High similarity between familial and sporadic cases of Chronic Lymphocytic Leukemia. ASH 2003 Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. 28) Rassenti LZ, Traci L. Toy, Lang Huynh, Andrew Greaves, John G. Gribben, Michael J. Keating, Kanti R. Rai, Ian W. Flinn, John C. Byrd, Neil E. Kay, Lynn R. Goldin, Neil E. Caporaso, Thomas J. Kipps. High Similarity between Familial and Sporadic Cases of CLL. ASH 2003 29) Liguang Chen, John Apgar, Lang Huynh, Teresa Giago-Mcgahan, Rassenti LZ, Thomas J. Kipps. Expression of ZAP-70 Is Associated with Enhanced Phosphorylation of p72Syk, BLNK, and Phospholipase C Following B Cell Receptor Ligation in Chronic Lymphocytic Leukemia. ASH 2003 30) Roman Rieger, Mark J. Cantwell, Rassenti LZ, Charles E. Prussak, Thomas J. Kipps. Cellular Immune Response to Ad-CD154 Immune Therapy for Chronic Lymphocytic Leukemia. ASH 2003 31) Mitsufumi Nishio, Frank Dicker, Rassenti LZ, Traci Toy, Howard B. Cottam, Dennis A. Carson, Michael Karin, Thomas J. Kipps. CLL Cells That Express Unmutated Immunoglobulin Genes Are Uniquely Sensitive to Inhibitors of Kappa B Kinase-beta (IKK). ASH 2003. 32)Mitsufumi Nishio, Danelle F. James, Thomas J. Kipps. Different Activation of the NF-B Canonical and Alternative Pathway by Tumor Necrosis Factor (TNF) Family Proteins, BAFF and APRIL, in Chronic Lymphocytic Leukemia (CLL). ASH 2003 33) Frank S. Dicker, Rassenti LZ, Thomas J. Kipps. Modulation of Cell Cycle-Regulated Genes Correlates with Late Sensitization to Fas-Mediated Apoptosis of CD40-Activated Chronic Lymphocytic Leukemia Cells. ASH 2003 34) Frank S. Dicker, Rassenti LZ, Thomas J. Kipps. CD40-Ligation Induces Latent Sensitivity to Death-Receptor-Mediated Apoptosis Via a p53-Independent Pathway in Chronic Lymphocytic Leukemia B Cells. ASH 2003 35) Rassenti LZ, Lang Huynh, Traci L. Toy, John G. Gribben, Michael J. Keating, Kanti R. Rai, Ian W. Flinn, John C. Byrd, Neil E. Kay, Andrew Greaves, Arthur Weiss, Thomas J. Kipps. Zap-70 Is a More Reliable Marker of Disease Progression Risk Than Immunoglobulin Mutation Status in Chronic Lymphocytic Leukemia. ASH 2003 36) Chen L, Widhopf G, Huynh L, Rassenti LZ, Rai KR, Weiss A, Kipps TJ. Expression of ZAP-70 is associated with increased B-Cell receptor signaling in chronic lymphocytic leukemia. Blood 2002 100:4609-4614 37)Januario E. Castro, Shinichi Kitada, Anissa Agadir, John C. Reed, Thomas J. Kipps. G3139Genasense Induces Apoptosis of Chronic Lymphocytic Leukemia Cells Via a Mechanism Dependent upon the Presence of Thymidine Nucleotides and Phosphorothioate Backbone and Not Antisense Bcl-2 Sequence. ASH Abstract 2003. 38)Chen, L., Widhopf, G., Huynh, L., Rassenti, L. Rai, K., Weiss, A., Kipps, T.J. (2002) Expression of Zap-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood 100(13):4609-4614. 39)Chen, L. Apgar, J., Huynh, L., Dicker, F., McGahan, T., Rassenti, L, Weiss, A., Kipps, T.J. (2005). ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood :105,20362041 Project 4 (Gribben) (n=12) 40)Battle TE, Frank DA. STAT1 mediates differentiation of chronic lymphocytic leukemia cells in response to Bryostatin 1. Blood. 2003;102:3016-24 41)Battle TE, Castro-Malaspino H, Gribben JG and Frank DA. Sustained complete remission of CLL associated with use of a Chinese herbal extract: case report and mechanistic analysis. Leuk Res. 2003;27:859-63. 42)Gricks C and Gribben JG "Cytotoxic T cell responses against immunoglobulin in malignant and normal B cells: Implications for tumor immunity and autoimmunity" Current Pharmaceutical Design 2003; 9:1889-903. 43)Gricks C, Zahrieh D, Zauls AJ, Drandi D, Gorgun G, Mauerer K, Neuberg D and Gribben JG. Differential regulation of gene expression following CD40 activation of healthy compared to leukemic B cells. Blood. In press 44)Krackhardt AM, Harig S, Witzens M, Broderick R, Barrett P, Gribben JG. T-cell responses against CLL cells: implications for immunotherapy.Blood2002100:167-173 Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. 45)Krackhardt AM, Witzens M, Harig S, Hodi FS, Zauls AJ, Chessia M, Barret P, Gribben JG. Identification of tumor-associated antigens in chronic lymphocytic leukemia by SEREX. Blood 2002 100:2123-2131 46)Battle TE, Wierda WG, Rassenti LZ, Zahrieh D, Neuberg D, Kipps TJ, Frank DA. In vivo activation of STAT1 following CD154 gene therapy for chronic lymphocytic leukemia is associated with clinical and immunologic response. Clinical Cancer Research 2003 9(6):2166-72. 47)Gullu Gorgun, David Zahrieh, Daniela Drandi, Donna Neuberg, John Gribben. Gene Expression Profiling Reveals Defects in Genes Regulating T Cell Diffentiation and Effector Pathways in T Cells in Patients with CLL. ASH Abstract 2003 48)Daniela Drandi, Charles Lee, Paola Dal Cin, John G. Gribben. Array-Based Comparative Genomic Hybridization Identifies Deletion at 14q32 as a New Prognostic Marker in Chronic Lymphocytic Leukemia (CLL). ASH Abstract 2003 49)Harig S, Witzens M, Krackhardt AM, Trojan A, Barrett P, Broderick R, Zauls AJ, Gribben JG. Induction of cytotoxic T-cell responses against immunoglobulin V region-derived peptides modified at human leukocyte antigen-A2 binding residues. Blood. 2001 Nov 15;98(10):2999-3005. 50)Trojan A, Schultze JL, Witzens M, Vonderheide RH, Ladetto M, Donovan JW, Gribben JG. Immunoglobulin framework-derived peptides function as cytotoxic T-cell epitopes commonly expressed in B-cell malignancies. Nat Med. 2000 Jun;6(6):667-72 51)Mauerer, K., Zahrieh, D., Gorgun, G., Li, Aihonh, Zhou, J., Ansen, S., Rassenti, L. Gribben, J., (2005) Immunoglobulin gene segment usage, location and immunogenicity in mutated and unmutated chronic lymphocytic leukemia. BJI: 129(4), 499-510. Project 5 ( Plunkett ) (n=16) 52) Gandhi, V., Plunkett, W., Weller, S., Du, M., Ayres, M., Rodriguez, Jr., C.O., Ramakrishna, P., Hodge, J.P.,Keating, M.J. Evaluation of the Combination of Nelarabine and Fludarabine in Leukemias: Clinical Response,Pharmacokinetics and Pharmacodynamics in Leukemia Cells, J. Clin. Oncol., 19:2142-2132,2001. 53) Gandhi, V. and Plunkett W. Combination strategies for purine nucleoside analogs, in “The Chronic LymphoidLeukemias”, Cheson, B. D., ed., Marcel Dekker, Inc., NY., pp. 195-208, 2001. 54) Gandhi V. and Plunkett, W. Cellular and clinical pharmacology of fludarabine. Clinical Pharmacokinetics,41:93-103, 2002. 55) Kantarjian, H. Gandhi, V., Kozuch, P., Faderl, S., Giles, F., Cortes, J., O’Brien, S., Ibrahim, N., Khuri, F., Du M., Rios, M.B., Plunkett, W., and Keating M.J. Phase I Clinical And Pharmacology Study Of Clofarabine (ClF-ara-A; 2 Chloro-2’-Fluoro-Deoxy-9-beta-D-Arabinofuranosyladenine) in Patients with Solid and Hematologic Cancers, J Clin. Oncol, 21:1167-1173, 2003. 56) Huang, P., Feng, L., Oldham, E. A., Keating, M. J., and Plunkett, W. Superoxide dismutase: a novel target for selective killing of cancer cells. Nature, 407:390-395, 2000 57) Huang, P., Sandoval, A., van den Neste, E., Keating, M.J., and Plunkett, W. Inhibition of RNA transcription: a biochemical mechanism of action against chronic lymphocytic leukemia cells by fludarabine. Leukemia, 8:1405-1413, 2000 58) Hileman EO, Achanta G, and Huang P. Superoxide dismutase: an emerging chemotherapeutic target. Expert Opinion on Therapeutic Targets 5:697-710, 2001. 59) Carew, J.S., Zhou, Y., Albitar, M., Keating, M.J., Huang, P. Mitochondrial DNA Mutations in Primary Leukemia Cells after Chemotherapy: Clinical Significance and Therapeutic Implications. Leukemia, 17:1437-1447, 2003. 60) Pelicano, H., Zhou, Y., Hileman, E. O., Feng, L., Plunkett, W. Keating, M. J., and Huang, P. Interfering of the mitochondrial electron transport: A novel strategy to enhance drug-induced apoptosis in leukemia cells through a free radical-mediated mechanism, J. Biol Chem, 278:3783237839, 2003 61) Zhou, Y., Hileman, E. O., Plunkett, W., Keating, M. J., and Huang, P. Free radical stress in chronic lymphocytic leukemia cells and its role in cellular sensitivity to ROS-generating anticancer agents. Blood, 101:4098-4104, 2003. Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. 62) Yamauchi, T., Nowak, B., Keating, M.J., and Plunkett, W. DNA repair initiated in chronic lymphocytic leukemia lymphocytes by 4-hydroperoxycyclophosphamide is inhibited by fludarabine and clofarabine. Clin. Cancer Res.,7:3580-3589, 2001. 63) Yamauchi, T., Keating, M.J., and Plunkett, W. UCN-01 (7-hydroxystaurosporine) inhibits DNA repair and increases cytotoxicity in normal lymphocytes and CLL lymphocytes. Molecular Cancer Therap., 1:287-294, 2002. 64) Rao, V.A. and Plunkett, W. Activation of p53-mediated apoptotic pathway in quiescent lymphocytes after inhibition of DNA repair by fludarabine. Clin. Cancer Res., 9:3204-3212, 2003. 65) Balakrishnan, K, Stellrecht CM, Genini D, Ayers, M, Keating MJ, Leoni LM, and Gandhi V. Bioenergetically Compromised and Transcriptionally Challenged CLL Lymphocytes Undergo Apoptosis with Halogenated ATP. Blood, Feb. 17, 2005; Epub prior to print. 66)Rong Chen, Michael J. Keating, Varsha Gandhi and William Plunkett: Transcription Inhibition by Flavopiridol: Mechanism of Chronic Lymphocytic Leukemia Cell Death. Submitted to Blood 67)Gandhi, V, Plunkett, W, Bonate, P, Du, M, Nowak, B, Lerner, S, Keating, M. Clinical and pharmocokinetic study of clofarabine in chronic lymphocytic leukemia (CLL): Strategy for treatment. Submitted to Blood Project 6 (n=12) 68) Maher Albitar, Kim-Anh Do, Marcella M Johnson, Francis J Giles, Iman Jilani1, Susan O’Brien, Jorge Cortes, Deborah Thomas, Rassenti LZ, Thomas J Kipps, Hagop M Kantarjian, Michael Keating. Free circulating sCD52 as a tumor marker in chronic lymphocytic leukemia and its implication on therapy with anti-CD52 antibodies. 2004. (in press in Cancer). 69) Rush LJ, Raval A, Funchain P, Johnson AJ, Smith L, Bernbea M, Te-Hui Liu, Heerema NA, Rassenti LZ, Liyanarachchi S, Davuluri R, Bryd JC, Plass C. Epigenetic Profiling in Chronic Lymphocytic leukemia reveals Novelo methylation Targets. Cancer Research 2004, 64:2424-2433 70)Kanti R. Rai, Nancy Driscoll, Dale Janson, Alyssa Echevarria, Manish Sheth, Archana Bhargava, Simon Vinarsky, Uzma Iqbal, Tarun Wasil, Bhoomi Mehrotra. Incidence of CLL and Other Cancers in Families of CLL Patients. ASH Abstract 2003 71)Carew, J.S., Zhou, Y., Albitar, M., Keating, M.J., Huang, P. Mitochondrial DNA Mutations in Primary Leukemia Cells after Chemotherapy: Clinical Significance and Therapeutic Implications. Leukemia, 17:1437-1447, 2003. 72)Susan M. O’Brien, John G. Gribben, Deborah A. Thomas, Maher Albitar, William Wierda, Alessandra Ferrajoli, Susan Lerner, Hagop M. Kantarjian, Thomas Kipps, Michael J. Keating, the CLL Research Consortium. Alemtuzumab for MRD in CLL. ASH Abstract 2003. 73)Thomas J. Kipps, Januario E. Castro, William Wierda, Michael J. Keating, Jan Bole, Jayne Meyer, Heather Roehrs, Lisa Bouchard, Vivian Yuan, Harjinder Chana, Lisa Hami, Mark Bonyhadi, Ronald J. Berenson, Mark W. Frohlich. A Phase I/II Trial of Xcellerated T Cells in Patients with Chronic Lymphocytic Leukemia (CLL). ASH Abstract 2003 74)Iman Jilani, Susan O’Brien, Hagop Kantarjian, Jitkaroon Caiyo, Francis Giles, Alessandra Ferrajoli, Michael Keating, Maher Albitar. Quantitative Measurement of CD52 on the Surface of CLL Cells before and after Treatment with Almetuzumab. ASH Abstract 2003 75)Iman Jilani, Susan O’Brien, Hagop Kantarjian, Jitkaroon Caiyo, Francis Giles, Alessandra Ferrajoli, Michael Keating, Maher Albitar. CD23 Quantification in Chronic Lymphocytic Leukemia and the Effects of Anti-CD23 Therapy on Its Levels. ASH Abstract 2003 76)Maher Albitar, Kim-Anh Do, Marcella M Johnson, Francis J Giles, Iman Jilani1, Susan O’Brien, Jorge Cortes, Deborah Thomas, Laura Z. Rassenti, Thomas J Kipps, Hagop M Kantarjian, Michael Keating.Free circulating sCD52 as a tumor marker in chronic lymphocytic leukemia and its implication on therapy with anti-CD52 antibodies. 2003 123(5) :850-7. 77)Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, Reed JC. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood 2002 Vol 99 –3 Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. 78)Stephanie R. Brockman, Tait D. Shanafelt, Sarah F. Paternoster, Danielle M. Nast, Heather C. Flynn, Nancy D. Bone, Susan M. Geyer, Carlo M. Croce, Robert L. Phyliky, Thomas E. Witzig, Neil E. Kay, Gordon W. Dewald. Chromosome anomalies detected by interphase fluorescence in situ hybridization: correlation with significant biological features of B-cell chronic lymphocytic leukaemia.Br J Haematol. 2003,121(2):287-95. 79)Michael R. Grever, Gordon W. Dewald, David M. Lucas, Donna S. Neuberg, Maureen E. Baird, Sarah F. Paternoster, Stephanie R. Brockman, Gerard Lozanski, Vassiliki Rizouli, John G. Gribben, Ian W. Flinn, Martin S. Tallman, Shinichi Kitada, John C. Reed, Louis M. Staudt, Elisabeth Paietta, John C. Byrd. ZAP-70 Protein Expression Varies by Interphase Cytogenetic Group and May Predict Disease Progression to Requirement of Treatment among Select Genetic Groups in Patients with Chronic Lymphocytic Leukemia (CLL). ASH Abstract 2003 CRC Bioinformatics (Core A) (n=2) 80)Greaves AW, Payne PR, Rassenti L, Kipps TJ. CRC Tissue Core Management System (TCMS): Integration of Basic Science and Clinical Data for Translational Research. AMIA Proceeding 2003 (poster abstract) 81)Payne PR, Greaves AW, Kipps TJ. CRC Clinical Trials Management System (CTMS): An Integrated Information Management Solutions for Collaborative Clinical Research. AMIA Proceedings 2003. CRC Biostatistics (Core B) (n=6) 82)Battle TE, Wierda WG, Rassenti LZ, Zahrieh D, Neuberg D, Kipps TJ, Frank DA. In vivo activation of signal transducer and activator of transcription 1 after CD154 gene therapy for chronic lymphocytic leukemia is associated with clinical and immunologic response. ClinCancer Res. 2003 Jun;9(6):2166-72. 83)Calin GA, Trapasso F, Shimizu M, Dumitru CD, Yendamuri S, Godwin AK,Ferrancin M, Bernardi G, Chatterjee D, Baldassarre G, Rattan S, Alder H,Mabuchi H, Shiraishi T, Hansen LL, Herlea V, Mauro F, Dighiero G, MovsasB, Rassenti L, Kipps T, Baffa R, Fusco A, Mori M, Russo G, Liu C-G,Neuberg D, Bullrich F, Negrini M, Croce CM. A germline stop mutation inARLTS1, a new member of the ADP-ribosylation factor family, isassociated with familial cancers and reduced in vivo apoptosis. NEJM 2005 21;352(16):1667-76. 84)Gorgon G, Holderried TAW, Zahrieh D, Neuberg D, Gribben JG. Chronic lymphocytic leukemia cells induce changes in gene expression of CD4 and CD8 T cells. In press, JCI. 85)Gricks C, Zahrieh D, Zauls AJ, Gorgun G, Drandi D, Mauerer K, Neuberg D,Gribben JG. Differential regulation of gene expression following CD40 activation of leukemic comparedto healthy B cells. Blood. 2004 Dec15;104(13):4002-9. 86)Lin CW, Manshouri T, Jilani I, Neuberg D, Patel K, Kantarjian H,Andreeff M, Estrov Z, Beran M, Keating M, Estey E, Albitar M.Proliferation and apoptosis in acute and chronic leukemias and myelodysplastic syndrome. Leuk Res. 2002 Jun;26(6):551-9. 87)Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, Neuberg DS, Flinn IW, Rai KR, Byrd JC, Kay NE, Greaves A, Weiss A, Kipps TJ.ZAP-70 is a stronger predictor of disease-progression risk than immunoglobulin mutations status in chronic lymphocytic leukemia. N Engl J Med. 2004 Aug 26;351(9):893-901. APPENDIX E. FISH Questionnaire I All questions pertain specifically to FISH studies for Common chromosome anomalies in CLL. I. Laboratory __________________ ____ Page APPENDIX CORE C- (UCSD) II. Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Equipment Microscope __ ___________________ Microscope Model ______ __________ Fluorescent bulb Wattage ________________________ FISH Filters ______________________ Microscope Objectives _________________ FISH imaging system___________________ III. IV. Prior experience Current standard method of FISH for common chromosome anomalies in CLL? ________ Number of specimens tested for common chromosome anomalies in CLL in 2003? ________ Number of specimens tested with D-FISH and ASS probes for Common chromosome anomalies in CLL in 2003? _____ At diagnosis, do you routinely perform conventional cytogenetics and FISH? _________ At diagnosis, do you routinely study blood? ___________, bone marrow __________, bone marrow and blood? __________ After treatment, do you routinely perform only FISH? ___________ After treatment, do you routinely study blood? ___________, bone marrow _________? Both bone marrow and blood? ________ Methodology Do you use colcemid or other spindle fiber inhibitor? ____________ Do you use hypotonic solution in preparing cells for FISH? _________ What is your fixative?_______________________ Do you use a Thermotron to prepare interphase nuclei for CLL FISH? ____ Do you use a hybrite in your FISH process? _________ Do you use a Coplin jar method in lieu of a hybrite in your FISH process? ______ Do you use slides with small circles to limit probe usage? __________________ Do you use charged microscope slides? _______________________ How much probe to you use for each slide? ____________ How many slides to you prepare for each specimen? ___________ What is your counter stain?________ V. Analysis Number of interphase nuclei usually analyzed per specimen? _____________ Normal cutoff for each probe for each anomaly detected?______________________________ What are your scoring criteria?___________ What do you do with nuclei that do not meet your scoring criteria? ___________ How many technologists score each specimen? ___________________________ How do you image to document your results? _____________ How long do you keep your images before discarding them?_ ________________ Are your images in jpeg, tiff or other?_________________________________ Page APPENDIX CORE C- (UCSD) V. VI. VII. Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Does your laboratory have access to an automated scoring system? _________ Would you use an automated scoring system in the clinical trial? ___________ Quality assurance Do you participate in a proficiency testing program?_____________ If you do participate in a proficiency testing program, which one?_______ Do you run a positive control with each specimen or batch of specimens?__________ Do you have an expectation that results of individual technologists match?________ Do you have a written procedure for FISH?______________________ How do you calculate the percent of abnormal nuclei?_________________ How do you evaluate staff competency?_____________________________ For those techs involved in this project, how many years/months of experience do they have scoring BCR/ABL FISH? ___________ For those techs involved in this project, are they NCA-certified (or Canadian equivalent)? ___ Describe other aspects, if any, of your quality assurance program?_________ Effort to do FISH studies Do you have workload recording information for your FISH assay for CLL? _____ Do you think we should include workload recording in the pilot studies?_________ If your laboratory sees an unusual FISH pattern, are you willing to circulate the sample/slides for the other laboratories to access? _________________________________ Data management and communication Do you maintain a computerized database for FISH for CLL? _______ If No, please describe how you maintain records of the results. _____________________ If Yes, please describe the following: What data base management program do you use? ______ Can you export FISH data directly from your database? ____ Is this a commercial product? _____ Is this a custom program? ____ If you have an automated spot analyzer, does it download data directly into your database or another database? No If yes, describe the database. ___________________________ Can your database accommodate new data fields that are unique to this study ? ____ Are your data FISH data for CLL submitted to a central Cooperative Group database? ___ If yes, describe the central database and how data are transferred. __________ Can the database produce data files in the following formats: Excel spreadsheet? ____ Access data base? ____ Fixed format ASCII file? ____ Other (specify): ______________________________________________ Is the database on a single computer (desktop or laptop), or on a network? _____ Describe the type of computer (e.g., Dell PC, Macintosh, Toshiba laptop) and operating system (e.g., Windows 2000, Windows NT). _____ Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Do you have access to the internet? Yes If Yes, what browser do you use (e.g., Internet Explorer, Netscape): _____ Can you send and receive data files using the internet? ______ Can you send and receive graphics files (e.g., jpeg, tiff) using the internet? ____ Comments_____________________________________________________________ APPENDIX F. FISH Data Collection Form Name of cytogeneticist submitting data ____________________ Name of cytogenetics laboratory _________________________ Result answer form for pilot study: chromosome 6 Challenge 1 2 3 4 5 6 Normal nuclei 200 100 50 74 100 100 Number of mainline abnormal nuclei 0 100 150 100 100 100 Signal pattern NA 1R2G 3R3G 1R2G 1R2G 1R2G Number of subclone #1 abnormal nuclei 0 0 0 26 0 0 Signal pattern NA NA NA 1R1G NA NA Total nuclei analyzed 200 200 200 200 200 200 Percent abnormal nuclei 0% 50% 75% 63% 50% 50% Number of nuclei not analyzed 50 71 34 54 71 71 7 8 9 200 50 200 0 150 0 NA 3R2G NA 0 0 0 NA NA NA 200 200 200 0% 75% 0% 50 34 50 Result answer form for pilot study: chromosome 11 Challenge 1 2 3 4 5 6 Normal nuclei 200 100 50 74 100 100 Number of mainline abnormal nuclei 0 100 150 100 100 100 Signal pattern NA 1R2G 3R3G 1R2G 1R2G 1R2G Number of subclone #1 abnormal nuclei 0 0 0 26 0 0 Signal pattern NA NA NA 1R1G NA NA Total nuclei analyzed 200 200 200 200 200 200 Percent abnormal nuclei 0% 50% 75% 63% 50% 50% Number of nuclei not analyzed 50 71 34 54 71 71 7 8 9 200 50 200 0 150 0 NA 3R2G NA 0 0 0 NA NA NA 200 200 200 0% 75% 0% 50 34 50 Result answer form for pilot study: chromosome 12 Challenge 1 2 3 4 5 6 7 8 9 Normal nuclei 200 100 50 74 100 100 200 50 200 Number of mainline abnormal nuclei 0 100 150 100 100 100 0 150 0 Signal pattern NA 1R2G 3R3G 1R2G 1R2G 1R2G NA 3R2G NA Number of subclone #1 abnormal nuclei 0 0 0 26 0 0 0 0 0 Signal pattern NA NA NA 1R1G NA NA NA NA NA Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Total nuclei analyzed 200 Percent abnormal nuclei 0% Number of nuclei not analyzed 50 200 50% 71 200 75% 34 200 63% 54 200 50% 71 200 200 50% 0% 71 50 200 200 75% 0% 34 50 Result answer form for pilot study: chromosome 13 Challenge 1 2 3 4 5 6 Normal nuclei 200 100 50 74 100 100 Number of mainline abnormal nuclei 0 100 150 100 100 100 Signal pattern NA 1R2G 3R3G 1R2G 1R2G 1R2G Number of subclone #1 abnormal nuclei 0 0 0 26 0 0 Signal pattern NA NA NA 1R1G NA NA Total nuclei analyzed 200 200 200 200 200 200 Percent abnormal nuclei 0% 50% 75% 63% 50% 50% Number of nuclei not analyzed 50 71 34 54 71 71 7 8 9 200 50 200 0 150 0 NA 3R2G NA 0 0 0 NA NA NA 200 200 200 0% 75% 0% 50 34 50 Result answer form for pilot study: chromosome 17 Challenge 1 2 3 4 5 6 Normal nuclei 200 100 50 74 100 100 Number of mainline abnormal nuclei 0 100 150 100 100 100 Signal pattern NA 1R2G 3R3G 1R2G 1R2G 1R2G Number of subclone #1 abnormal nuclei 0 0 0 26 0 0 Signal pattern NA NA NA 1R1G NA NA Total nuclei analyzed 200 200 200 200 200 200 Percent abnormal nuclei 0% 50% 75% 63% 50% 50% Number of nuclei not analyzed 50 71 34 54 71 71 7 8 9 200 50 200 0 150 0 NA 3R2G NA 0 0 0 NA NA NA 200 200 200 0% 75% 0% 50 34 50 Result answer form for pilot study: chromosome 14 Challenge 1 2 3 4 5 6 Normal nuclei 200 100 50 74 100 100 Number of mainline abnormal nuclei 0 100 150 100 100 100 Signal pattern NA 2R3G 2R3G 2R3G 1R1G2F 1R1G2F Number of subclone #1 abnormal nuclei 0 0 0 26 0 0 Signal pattern NA NA NA 1R1G NA NA Total nuclei analyzed 200 200 200 200 200 200 Percent abnormal nuclei 0% 50% 75% 63% 50% 50% Number of nuclei not analyzed 50 71 34 54 71 71 7 8 9 200 50 200 0 150 0 NA 2R3G NA 0 0 0 NA NA NA 200 200 200 0% 75% 0% 50 34 50 Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDIX G. Tissue Core Familial CLL Forms G1: CRC Tissue Core Sample Collection Study Patient Letter CRC Tissue Core Sample Collection Study Patient Letter Date _________ CRC Site_____________________ Name _______________________________ Address____________________________ Address _____________________________ __________________________________ ____________________________________ Phone_____________________________ Dear________________ Thank you for contacting us on concerning your interest on Familial CLL research at __________________. The CLL Research Consortium is conducting studies to find the cause and cure of CLL. Currently, we are studying families with 2 or more blood relatives with CLL. We believe that this is a crucial component in the understanding of CLL and hope that by studying affected families, we will eventually identify environmental and genetic risk factor that might predispose to CLL. For more information about CLL and this Research Consortium, please go to the CRC public website at www.cllresearch.org. Participation in the CRC studies is completely voluntary. All identifiable information and the research results will remain confidential. See the attached patient consent form for more details about patient confidentiality. If you agree to participate, please contact the CLL Research Consortium at 1 (877) FAM4CLL or 1 (877) 326-4255. Participation in this study requires you to go to your personal physician or the closest CRC Site, and follow the procedures below: 1) Call 1-888-309-8273, option #6 to register as a cash patient with the UCSD CRC. 2) Sign, and have a witness sign the enclosed patient consent form. 3) Complete the enclosed CRC Familial CLL questionnaire. 4) Bring the enclosed buccal cheek cell kit to your physician to obtain the buccal cheek cells. 5) Have your physician draw a complete blood count (CBC) with differential and platelets; then send the printed results to the address below. 6) Have your physician draw 5 yellow top tubes and send them at room temperature in a box with the buccal cheek cells kit, signed consent form, and completed questionnaire to : Dr. Laura Rassenti, Director CRC Tissue Core (Fed Ex use account # 245098120) UCSD Dept. Of Medicine (bill recipient) Stein Clinical Research Bldg. Room # 108C La Jolla, CA 92093-0663 Any questions, please contact: Marilyn Nolen, R.N., (Coordinator, CRC Familial CLL Studies) 1 (877) FAM4CLL or (858)-822-4966 (ph); (858)-822-5560 (fax); mnolen@ucsd.edu. Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Thank you for your interest and participation in this project, your contribution is invaluable and greatly appreciated by the CLL community. Sincerely, Laura Rassenti, PhD, Director CRC Tissue Core 858-534-5498 (ph) 858-534-5620 (fax) e-mail lrassenti@ucsd.edu G2: CRC Tissue Core Sample Collection Study Familial History Questionnaire First Name: ________________MI: ____ Last Name: ____________________ 1) Date of Birth: _______/____/_________ (MM/DD/YYYY) 2) Sex: Male Female 3) Race: White Black Asian Native Hawaiian Native American Patient Refusal Unknown 4) Ethnicity: Hispanic or Latino Non- Hispanic Unknown 5) Age at Diagnosis: _______ 6) Stage at Diagnosis (if known): ____________________________________ 7) Treatment(s) for CLL: ___________________________________________ ________________________________________________________________ 8) Other Cancer(s) Diagnosed (include date): _________________________ ________________________________________________________________ Family History Questions: 9) Family history of CLL (Chronic Lymphocytic Leukemia) Yes No Unknown 9a) If question 9 is YES, is the affected biological relative: (Please specify disease and # of affected, i.e. lymphoma, 2 brothers): Biological Mother _______________________ Biological Father _______________________ Brother (s) _____________________________ Sister (s) _____________________________ Son (s) _____________________________ Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Daughter(s) _____________________________ Other (s) _____________________________ 10) Family history of leukemia(s) or lymphoma(s) (blood cancer)? Yes No Unknown 10a) If question 10 is YES, is the affected biological relative (Please specify disease and # of affected i.e. lymphoma 2 brothers): Biological Mother _______________________ Biological Father _______________________ Brother (s) _____________________________ Sister (s) _____________________________ Son (s) _____________________________ Daughter(s) _____________________________ Other (s) _____________________________ 11) Family history of cancer other than of leukemia(s) or lymphoma(s) (blood cancer) ? Yes No Unknown 11a) If question 11 is YES, is the affected biological relative (Please specify disease and # of affected i.e. lymphoma 2 brothers): Biological Mother _______________________ Biological Father _______________________ Brother (s) _____________________________ Sister (s) _____________________________ Son (s) _____________________________ Daughter(s) _____________________________ Other (s) _____________________________ 12) Have you ever used tobacco products? (Cigarettes, chew, snuff, cigars, or pipes) Yes No 13) What is your highest level of education? Less than 8 years 8-11 years 12 years or high graduate post-high school other than college (vocational, technical) some college college graduate postgraduate other 14) Job held for the longest period : Retail Wholesale or distributor Service provider Manufacturing Construction Mining Farming, fishing, forestry Military Government Shipyard Other ___________________ 15) Are you a twin or one of a multiple birth? Yes No If ‘Yes’, Please Complete Questions 15a-c 15a. How many other infants were born with you? ___________ 15b. Of those born with you, how many are of an identical relation with you? __________ 15c. Of those born with you, how many are of a fraternal (non-identical) relation with you? _________ Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. G3: COLLECTION OF CHEEK CELL SAMPLES, CRC TISSUE CORE 1) Remove the swab from the package, do not touch the foam tip. 2) Place the foam tip in the patient’s mouth. Firmly move the swab around the inside of both cheeks. Use both sides of the foam tip to soak up as much saliva as possible. 3) Place the patient name and date of collection on the FTA card. 4) Open the FTA classic card. Firmly press, do not rub, the foam tip on two of the circles until wet. Continue pressing until both circles are saturated with saliva. Repeat step 2 and continue wetting the remaining 2 circles with the foam tip. 4) Allow the FTA card to air-dry at room temperature for 30min. 5) Place the FTA card inside the white foil-lined envelope and seal the envelope. 6) Place the white foiled-lined envelope into the Fed Ex envelope and mail it to : Dr. Laura Rassenti Director CRC Tissue Core Univ. of California, San Diego, Dept. of Medicine Stein Clinical Research Bld. Room # 108C La Jolla, CA 92093-0663 (858) 543-5498 e-mail lrassenti@ucsd.edu Alternatively, you can send these cards in the box with the blood samples The FTA coated paper is a matrix that protects the immobilized genomic DNA for long-term storage and PCR amplifications. G4: CLL General Information Flyer for Potential Familial Study Participants The Chronic Lymphocytic Leukemia (CLL) Research Consortium is a group of leading cancer research institutions dedicated to finding out more about this disease. Presently, the Consortium has a “bank” of thousands of patient blood and tissue specimens that scientists can ‘draw’ upon to conduct research studies. In the course of this research, we have found that some patients have blood relatives who also have CLL (“Familial CLL”), and believe it would be worthwhile to study the blood and tissue samples from members of the same family. If you are interested in finding out more about this project, you can call us toll free at: 1 877 FAM4CLL (1 858 326-4255), or view our website at www.cllresearch.org. Thank you for your interest and support. Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. APPENDIX H : Tissue Core Informed Consent Form & HIPAA Form H1: CRC Tissue Core Informed Consent Form (UCSD) CLL RESEARCH CONSORTIUM TISSUE CORE SAMPLE COLLECTION STUDY Dr. Thomas J. Kipps of the University of California, San Diego (UCSD) and the CLL Research Consortium (CRC) is formulating a national blood and tissue bank to receive, process, and store blood samples from subjects diagnosed with chronic lymphocytic leukemia, including individuals (Tissue Core Patients), as well as blood-relatives (Familial Subjects), who have CLL in an efficient and organized manner. This central bank will allow researchers within the CRC to find out more about the causes and biology of chronic lymphocytic leukemia. In addition, the investigators in the CRC are attempting to develop more effective treatments for this disease. It is estimated that 2000 samples will be obtained every year from participants across the nation and approximately 400 samples per year from UCSD. You have been asked to participate because you have chronic lymphocytic leukemia (CLL) and, in the case of Familial Subjects, have been identified as having a family member with CLL as well. If you agree to participate in this study, the following will happen to you: B. You will have up to 50cc (about 5 tablespoons) of blood drawn from your vein at intervals of three weeks or more when you are seen for routine clinical follow-up evaluation. The blood will be obtained in a similar manner to the collection of an ordinary blood sample drawn for a routine blood test. For convenience, these research samples may be drawn when you give blood for the routine blood tests that are necessary to monitor your disease. Also, if you have had blood, bone marrow, or tissue samples collected for medical reasons related to your leukemia, then you agree to allow for such samples to be examined, or to have the reports of such evaluated by the CRC for research purposes. This will not require any additional samples from you. You may refuse to donate research blood samples or to have your left-over blood or tissue samples donated to the CRC at any time without jeopardizing your clinical care. C. You will also have a cheek swab performed when you are in the clinic. This swab is similar to having a throat culture, except that the swab will be made from your inner cheek, instead of the back of your throat. This is to collect cheek cells for the purpose of harvesting their DNA. This DNA may be compared with the DNA of your leukemia cells to examine for differences that could represent mutations responsible for the development of your leukemia. 3. The CRC will periodically post updates, on our public website, describing the research of the CRC. We invite you to review these updates at www.cllresearch.org. Your blood and cheek swab sample, as well as samples that may have been collected previously, will be used in present and future research conducted by the CLL Research Consortium. Dr. Kipps and co-investigators in the CRC will be responsible for deciding how these samples will be used. Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. Dr. Kipps, his associates, or his successors may keep your specimens or DNA and the information derived from them indefinitely. The specimens collected from you, and the DNA that they contain, also may be used in additional research to be conducted by CRC personnel and collaborators. Although not anticipated, your blood, cheek swab, and other tissue samples, and their derivatives may have significant therapeutic or commercial value. You consent to such uses. If you decide later that you do not want the specimens collected from you to be used for future research, you may tell this to Dr. ______________, who will use his best efforts to stop any additional studies. However in some cases, it may be impossible to locate and stop such future research once your specimens have been widely shared with other researchers. Participation in this study may involve some risks or discomforts. These include: E. Mild pain when the needle enters your skin, slight bruising at the puncture site, dizziness, or occasional fainting. And as with any procedure that produces a break in the skin, you also will have an increased risk of developing an infection. However, if any new risks become known in the future you will be informed of them. F. Although a number of factors may contribute to the risk of developing CLL, some of these may be cancer susceptibility genes that can be carried from one generation to the next in certain families. Although currently there are no genetic tests that identify this disease, current and future CRC studies are attempting to identify these risk factors and may result in genetic tests that help identify people with increased risk for CLL. Because there are no absolute legal protections against discrimination on the basis of genetic information, the CRC will treat all studies using these specimens as research only. Instances are known in which a patient has been required to furnish genetic information as a precondition for application for health insurance and/or a job. Participation in this study does not mean that you have had genetic testing. Should such a clinical test be developed, then this will be posted on the CRC website. Research laboratories that test your blood, cheek swab, and tissue samples will not be given identifying information about you. Information about your participation, your identifier code, and the complete research results on your blood or tissue samples will be stored together only at the Administrative and Tissue Core offices of the CRC at the University of California, San Diego. The research information obtained from the study of your blood or tissue samples will not be placed in your medical records. Although measures will be taken to ensure the confidentiality of your samples, there is a risk that this confidentiality will be broken, resulting in release of information that links your name with the results obtained from the research analyses performed on your tissue samples. If you are injured as a result of participation in this research, the ____________(CRC Site)____________ will provide the medical care needed to treat those injuries. The __________(CRC Site)________ will not provide any other form of compensation to you if you are injured. You may call the _________(CRC Site)_____________ IRB Office at (XXX) XXX- Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. XXXX for more information about this, to inquire about your rights as a research subject, or to report research-related problems. There will be no direct benefit to you from this study since you will not be provided with any results or the information obtained from testing your samples. The investigators, however, may learn more about B cell CLL and develop more effective therapies for this disease. Participation in research is entirely voluntary. You may refuse to participate or withdraw at any time without jeopardizing the medical care you receive at this or any other medical institution. The investigator also may stop this study at any time. Dr.____________________________ has explained this study to you and answered your questions. If you have other questions or research-related problems you may reach ______(CRC Site Research Nurse)_____, RN at (XXX) XXX-XXXX. Research records will be kept confidential to the extent provided by law. You have received a copy of this consent document to keep and a copy of “The Experimental Subject’s Bill of Rights.” You agree to participate. _______________________________________ Subject’s Signature ______________________________ Date _______________________________________ Witness’ Signature ______________________________ Date Page APPENDIX CORE C- (UCSD) Principal Investigator/Program Director (Last, first, middle): Kipps, Thomas J. G2: HIPAA FORM Authorization for Use of Protected Health Information for Research Purposes (HIPAA) Addendum for CLL Research Consortium Tissue Core Sample Collection Study Consent You have been asked to be part of a research study under the direction of Dr.________________ and the CLL Research Consortium. The purpose of this study is to gather blood and tissue samples from patients with CLL so that researchers can learn more about the causes and biology of Chronic Lymphocytic Leukemia. By signing this document, you authorize the use for research purposes of the following information about you - medical records of your treatment for CLL - laboratory test results If you do not sign this authorization, you will not be part of the study. This authorization has no expiration date. You can revoke this authorization at any time. To revoke your authorization, you can write to Dr. ______________or you can ask a member of the research team to give you a form to revoke the authorization. If you revoke this authorization, you will not be able to continue to participate in the study. However, this will not affect your rights to receive health care at _________________. If you revoke this authorization, Dr. ________________ and his or her research team can continue to use information about you that has already been collected. No information will be collected after you revoke the authorization. This study includes the creation of a database of information or specimens such as blood, tissue, or other bodily fluids that will be used in future research. By signing this authorization, you agree to allow the information collected in this study to be added to that database. The _________(CRC Site)___________and the CLL Research Consortium comply with the requirements of the Health Insurance Portability and Accountability Act of 1996 and its privacy regulations, and all other applicable laws that protect your privacy. We will protect your information according to these laws. Despite these protections, there is a possibility that your information could be used or disclosed in a way that it will no longer be protected. Our Notice of Privacy Practices (a separate document) provides more information on how we protect your information. If you do not have a copy of the Notice, the research team will provide one to you. By signing this authorization you agree that you have read this authorization form and have been given the opportunity to ask questions. If you have questions later, you I can contact [insert contact person name]. You will be given a signed copy of this authorization for your records. You authorize the use of your health information as described in this form. ______________________________ Participant signature ________________ Date Page APPENDIX