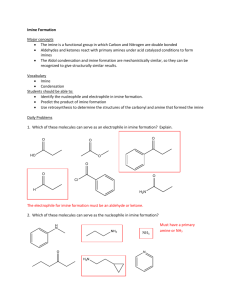
Study on tautomerism reaction of p-amino benzylidene-p
advertisement

Study on tautomerism reaction of p-amino benzylidene-p-hydroxy aniline by UV spectroscopy A.S.P.Azzouz and A.B.N.Al-Dabagh Chemistry Department , College of Education , University of Mosul , Iraq Keyword: Schiff base , Cis-trans isomers , tautomerism , equilibrium constant . Abstract p-Amino benzylidene-p-hydroxy aniline (I) was synthesized from paminobenzaldehyde and p-aminophenol by a standard method . Its structure was confirmed by chemical and physical methods . The former method included , by using specific reagents for phenol and primary amino groups . The latter method performed by the measurements of melting point and UV-IR spectra of I . Originally , the project was designed to study stability constants of azo dye complexes formed by reactions of I with diazotized sulphanilic reagent and other similar reagents . The unsuccessful study for complex formation stated last , encourage the workers in this investigation to shift the direction of project toward a clear answered for the reason of impossible determination of stability constant value for the dye stated . Hence the tautomerism study which happened in I in basic medium with their experimental factors , such as pH , temperature and thermodynamic parameters were outlined in this investigation . Introduction Tautomerism was an important area of chemical study1 , that had long been challenge computationally , experimentally and intellectually . Tautomerism interconversions2 had been investigated by chemists during last decades . Tautomerism study had received renewed attention due to its importance3 in determination of compound properties and their area of applications . Such topic was regarded as a major topic in theortical chemistry . For example tautomerisms of keto-enol4-5 , imine-enamine6-7 and many other systems7 . 1 The concept of tautomerism of carbonyl8 , analide9 , Schiff bases10 and benzoin11 had been extensively studied . As a continuation of tautomerism reactions11-13 , performed in our laboratory . This work describes the influence of alkali as sodium hydroxide on UV spectrum of p-amino benzylidene-p-hydroxy aniline , which led to tautomerism reaction . The work describs the influence of buffer solutions and temperature in the range 3-10 and (283-333)K respectively , on tautmerism reaction in Schiff base under study . Experimental Pure p-amino benzaldehyde and p-hydroxy aniline were supplied from Fluka chemical company . They were converted to Schiff base I by using a standard method14 , or by mixing equimolar amounts of p-amino benzaldehyde and p-hydroxy aniline in 20ml absolute ethanol . The product collected was recrystallized from ethanol , yield a product having a melting point of (168-170)ºC . Optimal conditions for tautomerism of imine I The proper solvent used in tautomerism study of I , was a mixure of 20% acetone – 80%ethanol . Originally , a 5×10-5M of solution I in a solvent stated was prepared , followed by measurement of its UV spectrum against a blank sample . Then after , to this solution, either different 0.5M and 1M of Na2CO3 or 1M NaOH were added , followed by measurements of their UV spectra in a similar way . Preparation of buffer solutions The following buffer solutions were used in tautomerism study as in Table (1). Table (1) : Compositions of buffer15 in the pH range 4-10 pH 4 5 6 7 8 9 10 Composition 0.2M CH3COONa (9ml) + 0.2M CH3COOH (41ml) 0.2M CH3COONa (32.25ml) + 0.2M CH3COOH (14.75ml) 0.2M CH3COONa (19ml) + 0.2M CH3COOH (9ml) 0.05M Borax (12ml) + (12.4g Boric acid +2.93g NaCl in liter)(188ml) 0.05M Borax (11ml) + (12.4g Boric acid +2.93g NaCl in liter)(29ml) 0.05M Borax (40ml) + (12.4g Boric acid +2.93g NaCl in liter)(10ml) Equal Volume from 0.025M (Na2CO3 +NaHCO3) 2 Effect of buffers on tautomerism of I In order to confirm the tautomerism of I , a similar 1ml of 10-4M of imine I in the mixed solvent stated was prepared and diluted to 10ml with any buffer solutions . The UV spectrum for each study was measured versus blank solvent . Effect of temperature on tautomerism of I A similar study was adopted here to the last by measurements of UV spectra at a temperature range between (10-50)ºC . The last resulted to different values of equilibrium constants , followed by linear plots shown later using Microsoft Excel 2007 . Instrumentations 1. The m.p of imine I was measured by using 30 SMP apparatus manufactured by Bibby scientific limited 2003 . 2. The UV spectra was measured by using Shimadzo UV-1650 spectrophotometer using a quartz cells of dimensions 1×1×3 cm3 . This connected to a water bath model EyEL4 type NTT-2200p , manufactured by Rikakikai Co. Ltd. Tokyo. 3. The IR spectra was measured for solid sample by using FT-IR spectrophotometer Tenser-27 Bruker company by KBr disk method . 4. The pH of any solution was measured by WTW 82362-Weilheim pH720 apparatus . Results and Discussion The chemical structure of imine I was confirmed by using similar physical method , namely by using melting point and UV-IR spectra . Results collected here were compared with other salicylidene ortho meta and p-hydroxy anilines whose had similar structures and were well agreed16 with our study . A preliminary study was started by the measured of UV spectrum of I in mixed 20% acetone-80% ethanol solvent as in Fig. 1 which showed the following absorption bands as Table 2 . 3 Fig. 1 : Original UV spectrum of 5×10-5M of imine 1 in 20% acetone-80% ethanol These bands have molar extension coefficient values of more than 1000 units and confirmed three π π* transitions resulted from three different chromophoric linkages, namely two aromatic rings and one azomethine linkage . This was in disagreement with EL-Bayoumi17 finding on benzylidene aniline who has similar structure to imine I . These variation of results could be interpreted due to the presence of NH2 and OH substituents on para positions of aromatic rings of imine I , which could cause such expected different results . A previous study13 showed that addition of NaOH solution to 2-hydroxy-1-napthylaldehyde resulted to zwitter ion formation that led to keto tautomer by resonance process . On the same basis the workers added solutions of 0.5M and 1.0M base Na2CO3 and 1.0M NaOH to 0.5×10-5M of I in 20% - acetone 80% ethanol mixed solvent as in Fig. 2 which showed the following data :Table 2 : Absorption spectra of imine I in 80% acetone – 80% ethanol mixed solvent a) Original spectrum b) After addition of bases Conc.of base a- λ(nm) 333.0 250.0 4 A 1.188 0.818 Σmax Liter.mole-1.cm-1 23.76×103 16.36×103 0.5M Na2CO3 b- 1M Na2CO3 1M NaOH 234.0 321.0 259.6 251.0 242.0 318.8 259.0 250.2 246.2 320.4 259.6 251.4 243.0 0.248 1.057 0.919 0.983 0.972 0.724 0.591 0.644 0.669 0.704 0.668 0.708 0.680 4.96×103 21.14×103 18.38×103 19.66×103 19.44×103 14.48×103 11.82×103 12.88×103 13.38×103 14.08×103 13.36×103 14.16×103 13.60×103 Table 2 showed three UV bands of original spectrum , had completely and suddenly changed to four UV bands after addition of Na2CO3 and NaOH bases . In literature18-19 it was confirmed that cis and trans keto tautomers appeared at longer wavelength as compared with enol tautomer . Hence , it was suitable to mentioned in this respect , that UV band appeared after addition of bases as a result of tautomerism reactions in imine . These appeared tautomers in imine I in a direction of increasing wavelengths were in order of trans keto , cis keto , enol and imine respectively . These tautomerism reactions happened in I in the presence of bases were in agreement20-21 and with mechanism of base catalysed21 reactions , as shown in Scheme I . Influence of buffers in the range of pH 4-10 on tautomerism of I In order to certain the mentioned tautomerism reactions happened in I , this led to the preparation 10-4M solutions of imine in a buffers having pH range 4-10 in the same mixed solvent . Results collected were tabulated in Table 3 . Table 3 : UV absorption bands of 10-4M imine I at buffers having pH range 4-10 . pH Natural 4 λ(nm) 328.4 231.6 412.6 321.6 224.0 A 0.438 0.365 0.202 0.573 0.360 5 Σmax Liter.mole-1.cm-1 4380 3650 2020 5730 3600 5 7 8 10 412.2 322.0 232.6 230.8 323.8 325.8 234.2 327.4 234.6 0.202 0.564 0.446 0.536 0.351 0.625 0.478 0.701 0.567 2020 5640 4460 5360 3510 6250 4780 7020 5670 Table 3 showed the following results :1. The original UV spectrum of imine I having two bands in equilibrium with each other . These related to the presence of I in enol and keto forms with extinction values of 4380 and 3650 Liter.mole-1.cm-1 respectively . The greater Σmax value for enol as compared with keto means an increase abundance of enol tautomer over keto tautomer in solution . 2. At pH values 4, 5 imine I showed another third band at wavelength 412.6nm or 412.2nm with molar extension value of 2020 . This attributed to the formation of nitriliun or oxonium ion as as result of combination10 of H+ with nitrogen azomethine or with oxygen of phenol group in imine . The rest of UV bands were related to the occurance of I in enol and keto forms , with an increase in concentration of enol as compared keto at these two pH values . 3. At pH7 , imine I showed two UV bands for enol and keto forms with Σmax values of 5360 and 3510 respectively . This was in agreement with upper previous paragraph . 4. Imine I had shown two UV bands at pH 8 or 10 related to keto and enol tautomers respectively . This last statement was confirmed with bathochromic shift of keto band into a range of wavelength (325.2-327.4)nm and the appearance of new extinction coefficient values of 7020 and 6250 Liter.mole-1.cm-1 for keto and enol tautomers respectively . In summary statements , at pH 8 or 10 the tautomerism of I enol to its cis and trans keto tautomers . 6 )a) )b) )c) Fig. 2 : UV spectrum of 5×10-5M after addition of :a- 0.5M Na2CO3 , b- 1M Na2CO3 , c- 1M NaOH 7 CH H H 2N O H +OH N C N K3 Enol-Amine NH2 K2 O O Cis keto K4 H NH 2 C N CH HN Trans keto N OH Imine Scheme I : Mechanism of tautomerism reactions in imine I after addition of a base Influence of temperatures on tautomerism of I In this respect a mixture from 5×10-5M from I with 0.5M Na2CO3 in the mixed solvent mentioned , was prepared . The UV spectra for mixture were recorded at temperature range (10-50)ºC ,versus blank sample . The record spectrum was shown in Fig. 2a , having four peaks , as illustrated in Table 4 . Table 4 : Absorbances of mixed imine with Na2CO3 at temperature range (10-50)ºC tc0 A1 1nm A2 2nm A3 3nm A4 4nm 10 0.840 251 0.300 334.6 0.581 259.6 0.604 242.2 20 0.782 251 0.460 323.0 0.601 259.8 0.629 242.2 30 0.728 251 0.551 321.4 0.671 259.6 0.659 242.2 40 0.655 251 0.700 321.4 0.708 259.8 0.669 242.2 50 0.590 251 0.718 321.2 0.767 262.4 0.710 242.2 These four wavelengths λ1 , λ2 , λ3 and λ4 were related to tautomers enol , trans keto , cis keto and new imine product tautomer respectively . Actually , the last tautomer was formed by tautomerism of type eneamine imine reaction . These λ1 , λ2 , λ3 and λ4 having absorbances values A1 , A2 , A3 and A4 respectively . When the last absorbances were plotted versus the temperature (t) resulted to a linear plots with correlation coefficient range values R2 = 0.955 – 0.9971 . 8 a b c d Fig. 3 : The relationships between absorbances versus t for a) A1 versus t b) A2 versus t c) A3 versus t d) A4 versus t 9 Careful examination of plots showed an inverse relation-ship between reactant absorbance A1 for enol tautomer with temperature , where as products absorbances A2 , A3 and A4 showed a direct relationships . These results were expected and agreed with practical experiments . The thermodynamic parameters ΔGº , ΔHº and ΔSº for tautomerism reactions of I as shown in Sheme 1 , were estimated by using Hartman22 etal for tautomerism reaction as an example A1 A2 . Hence the equilibrium constant K can be calculated by equation (1) K = C2/C1 = A2/A1 . α1/ α2 … (1) C1 and C2 = concentrations of reactant and product in the equilibrium reaction . A1 and A2 =Absorbances of reactant and product respectively . α1 and α2 = molar extension coefficient of reactant and product respectively . CT = Total concentration of reactant . CT = C1 + C2 … (2) , Applying Beer law to (2) d = path length of cell . In a previous study22 , it was assumed that α1 and α2 values do not changed by the variation of temperature . When the three equilibrium reactions shown in Scheme (1) applied to equation (4) , the results were a three plots outlined in Fig. 4 of R2 range values 0.9442-0.968 with values of α1/ α2 , α1/α3 equal 0.5541 , 1.7039 and 0.3998 respectively . 10 a b c Fig. 4 : The relationships of tautomerism reactions of I as :a) A1 versus A2 b) A1 versus A3 c) A1 versus A4 The equilibrium constants K2 , K3 and K4 in imine I were calculated as shown in Table 3 . The variation of K2 , K3 and K4 with temperatures , facilitate the evaluation of enthalpy ΔHº for tautomerism reactions using integrated Vant Hoff equation of the form :11 The plots of lnK2 , lnK3 and lnK4 versus T-1 showed a linear plots as in Fig. 5 . a b c Fig. 5 : In relationship of lnK versus T-1 for :a) lnK2 versus T-1 b) lnK3 versus T-1 c) lnK4 versus T-1 12 The rest of thermodynamic parameter ΔGº and ΔSº were calculated from equations (6-7) of the forms :ΔGº = ΔHº - ΔSº …(7) Table 5 : The thermodynamic parameters ΔGº , ΔHº and ΔSº for tautomerism in I G20 G2 KJ.mole-1 KJ.mole-1 H 20 H 2 KJ.mole-1 KJ.mole-1 S 20 S 2 J.mole-1.K-1 J.mole-1.K-1 1 T A K2 2 . 1 A1 2 lnK2 0.0035 0.1978 -1.6204 3.81257 19.26688 54.60 0.0034 0.3259 -1.1211 2.73100 18.73139 54.60 0.0033 0.4193 -0.8691 2.18938 2.23091 18.73586 0.0031 0.5921 -0.5240 1.36359 18.45616 54.60 0.0030 0.6743 -0.3940 1.05805 18.69671 54.60 1 T A K3 3 . 1 A1 3 lnK3 0.0035 1.1785 0.1642 -0.386.33 10.02295 36.78 0.0034 1.3095 0.2696 -0.65674 10.12037 36.78 0.0033 1.5704 0.4513 -1.13688 0.0031 1.8417 0.6107 -1.58921 9.92354 36.78 0.0030 2.2150 0.7952 -2.13544 9.74512 36.78 1 T A K4 4 . 1 A1 4 lnK4 0.0035 0.2874 -1.2468 0 G30 G3 KJ.mole-1 KJ.mole-1 0 -1.18092 0.3215 -1.1347 18.77740 H 30 H 3 KJ.mole-1 KJ.mole-1 10.00804 0 9.96400 54.60 0 54.60 S 30 S 3 J.mole-1.K-1 J.mole-1.K-1 36.78 0 36.78 G40 G40 H 4 H 40 S 40 S 4 KJ.mole-1 KJ.mole-1 KJ.mole-1 KJ.mole-1 J.mole-1.K-1 J.mole-1.K-1 2.93354 7.936.67 2.51067 0.0034 0 2.76413 13 17.67 7.86737 7.94404 0 17.67 17.67 0.0033 0.3619 -1.0163 2.56020 7.91690 17.67 0.0031 0.4083 -0.8957 2.33086 7.86435 17.67 0.0030 0.4811 -0.7316 1.96465 7.67493 17.67 The positive signs of all parameter means that all three tautomerism reactions of I were enothermic or occurred after absorption of heat . has a negative signs . This means that tautomerism reaction of k 3 equilibrium constant occurred spontaneously . and positive signs as explained by a non spontaneous reactions performed . They have equilibrium constants values less than unity . have to be Finally all values of , and have a positive signs . These mean that all tautomerism reactions resulted in more random of products . References 1. M.R.J. Hachey, spectroscopy, 2004, 19(4), 14 . 2. J.Grochowski, P.Serda, M.Markiewicz, B.Kozik and J.Sepiol, J.Mol. Struct. (theochem.), 2004, 689, 43 . 3. M.Shabanian, H.Moghanian, M.Hajibeygi an.1 A.Mohamadi, EJournal of Chemistry, 2012, 9(1), 107 . 4. A.Misra and S.Dalai, J.Mol Struct.(Theochem), 2007, 807, 33 . 5. K.Zborowski and A.Korenova, Theochem. 2004, 683,15 . 6. W.P.Oziminskj, J.C.Dobrovolski and A.P.Mazurek, J.Mol. Struct. (Theochem.), 2004, 680, 107 . 7. J.A.Bonacin, A.L.B.Formiga, V.H.S. de Melo and H.E.Toma, Vib Spectrosc., 2007, 44(1), 133 . 8. Ed.Z.Zabicky, The Chemistry of carbonyl group, 1970, Interscience, London, 157 . 9. G.Allen and R.A.Dwek, J.Chem.Soc., 1966, 161 . 10.A.S.P.Azzouz and A.B.N.Al-Dabagh, Nat.J.Chem., 2007, 26, 295 . 11.A.S.P.Azzouz, M.A.Hussin, M.G.Al-Dabbagh, Nat.J.Chem., 2010, 38, 361 . 12.A.S.P.Azzouz ,Mutah J.Res., and Stud., 1993, 8, 93 . 14 13.A.S.P.Azzouz, A.A.Rahman, and A.G.Taki, Nat.J.Chem., 2005, 20, 568 . 14.A.I.Vogel, Text Book of Practical Organic Chemistry, 3rd ed., 1956, London, Longman, 344 . 15.A.Findlay and J.A.Kitchener, Practical Physical Chemistry, 8 th ed., 1963, Longmans, P.268 . 16.A.S.P.Azzouz, T.S.Al-Ghabsha and A.N.Obed Agha, J.Kirkuk University, 2012, 7(2), 15 . 17.M.A.El-Bayoumi, M.El-Asser and Abdel-Halim, J.Amer.Chem.Soc., 1971, 93, 586 . 18.R.S.Becker and W.F.Richey, J.Amer.Chem.Soc., 1967, 89,1298 . 19.Ed.S.Patai, The Chemistry of Carbon Nitrogen Double Bond, 1979, John Wiley and Sons, NewYork, PP.50, 61, 65 . 20.Encyclopedia Britannica Inc. Web <http://www.britannica.com, 2012 . 21.A.N.Nesmeyanor and N.A.Nesmeynov, Fundamental of Organic Chemistry, 1981, vol.2 Mir publisher, Moscow, pp.73-80 . 22.K.O.Hartman, G.L.Carlson, R.E.Witkowiski and W.E..Fateley, Spectrochimica Acta, 1968, 24A, 158 . 15