Supplement lab safety rules
advertisement

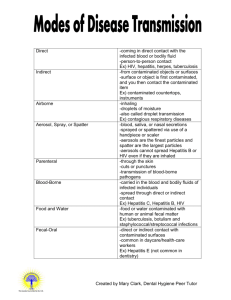
SUPPLEMENTAL SAFETY RULES FOR LABS WORKING WITH MICROORGANISMS (Biological Sciences Department) ALL MICROORGANISMS SHOULD BE TREATED AS POTENTIAL PATHOGENS EATING/DRINKING IS PROHIBITED IN ALL LABS. Lab benches should be kept clear of coats, books, or other personal items. Keep only those items on the lab bench that are needed to perform experiments. The lab bench area should be cleaned with disinfectant solution before you begin work and again before you leave the area. Hands should be thoroughly washed with germicidal solution before starting lab work and after the day's lab work has been completed. A full-length lab coat must be worn at all times to protect yourself and clothing from contamination or accidental discoloration by staining solutions. Mouth pipetting is strictly forbidden. Use mechanical pipetting devices or rubber bulbs. Always discard disposable pipet tips and glass pipets into designated pipet collection jars containing a disinfectant solution. Avoid placing items into your mouth during lab courses. Items such as pencils, lab markers, tape and fingers may have come in contact with infectious material. Report all spills at once to your instructor. Cover spills with paper towels that have been soaked with a disinfectant. Keep in place for at least 15 minutes before discarding in a biohazard bag. In the event of a major spill which cannot be contained by you or your instructor, notify the PrepRoom staff at extension 4070/4652. If the Prep-Room staff is not available, notify the Dept. Health & Safety Technician at extension 3649. Do not place contaminated materials such as inoculating loops, needles, and pipettes on tabletops. Loops and needles should be sterilized by incineration (flaming). Used pipets should be disposed of in designated receptacles. Never discard contaminated liquids or liquid cultures into the sink for disposal. Put them into designated collection containers in the lab for sterilization after the lab period. Upon completion of the laboratory session, discard all cultures and materials in designated discard areas or containers. Place uncontaminated broken glassware in the separate blue and white "Broken Glass" cardboard boxes. Any broken glassware that is contaminated with microorganisms must be placed in a biohazard collection container for decontamination by autoclaving before being discarded. Cultures, media, and equipment should never be taken from the laboratory unless directed by lab instructor. Keep culture tubes in a test-tube rack whenever possible, especially when carrying cultures around the lab and when working on the benchtops. All technical procedures should be performed in a manner that minimizes the creation of aerosol that may contain infectious material. My signature on the Signature Sign-up Sheet certifies that I have read and understood the above laboratory safety rules and regulations and agree to abide by them. (If a copy of this document is not printed in your lab manual or course syllabus (lab hand-outs), please ask for a personal copy for your records) Lab Safety Sheet Series (Part 3: 2 pages) Revised 3/05 1 of 2 Approved by Dept. Safety Committee and Cal-Poly EH&S DISCARD PROCEDURES (The following containers for discards are available in most Micro labs) BASKETS — Used for discarding tubes of media (small and large). Prior to placing in baskets, students should remove lab marker labels from tubes using cotton saturated with acetone. Any tape on tubes or glassware should also be removed. BINS — One bin designated for plastic Petri dishes and two bins designated for glassware (contaminated and non-contaminated). SHARPS CONTAINER — Used for needles, syringes, blades, Pasteur pipets and slides. LARGE RED (RIGID) STEP-ON CONTAINER— Red bags used for solid contaminated materials such as gloves and paper towels. DO NOT PLACE LIQUIDS, SHARPS OR GLASS OF ANY TYPE IN RED BAGS! BROKEN GLASS CARDBOARD BOXES — Used for uncontaminated discarded and broken glass only! NO TRASH OR CONTAMINATED ITEMS! MICROSCOPE CHECK LIST When putting away the microscope: If you have used oil, carefully blot (DO NOT WIPE) oil from the objective and condenser lenses with LENS PAPER; never with Kim wipes. Align low-power (10 x) dry objective with condenser. Turn off light and turn light intensity to lowest position. Rack the condenser all the way up and then a half a turn down. Align mechanical stage parts with right edge of stage. Do not leave parts extended beyond stage. Wrap up cord securely. Carry the instrument to its storage location with one hand under the base and the other hand on the arm of the microscope. Lab Safety Sheet Series (Part 3: 2 pages) Revised 3/05 2 of 2 Approved by Dept. Safety Committee and Cal-Poly EH&S