17_Single Double Displacement v2
advertisement

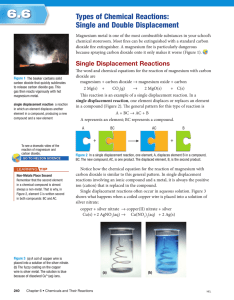
SNC2D – Chemistry: Chemical Reactions Notes: Types of Chemical Reactions II 1. Synthesis (Combination) 2. Decomposition 3. Single Displacement Definition/Format: Example/Practice: 3. Single Displacement 4. Double Displacement 5. Combustion SNC2D – Chemistry: Chemical Reactions 4. Double Displacement Definition/Format: Example/Practice: 5. Combustion Definition/Format: Example/Practice SNC2D – Chemistry: Chemical Reactions Single Displacement and Double Displacement Practice Exercises: Section 1. For each of the chemical reactions listed below, complete the following: The type of chemical reaction (single or double displacement) Balance the skeletal equation 1. Sulphuric acid reacts with iron (II) sulphide to produce iron (II) sulphate and hydrogen sulphide. Reaction type: ___________________________________ Balance the skeletal equation: ______H2S04 + _____FeS _____FeSO4 + _____H2S 2. An alkali metal such as sodium displaces hydrogen from water to form sodium hydroxide and hydrogen gas. Reaction type: ___________________________________ Balance the skeletal equation: ______Na + _____H2O _____NaOH + _____H2 3. Valuable silver can be recovered from a solution of silver nitrate by adding copper to produce copper (II) nitrate and a silver precipitate. Reaction type: ___________________________________ Balance the skeletal equation: ______AgN03 + _____Cu _____Cu(NO3)2 + _____ Ag 4. If we were to add table salt to a solution of silver nitrate we would produce sodium nitrate solution and silver chloride. Reaction type: ___________________________________ Balance the skeletal equation: _____NaCl + ____AgNO3 ____NaNO3 + ____AgCl 5. Potassium iodide reacts with lead (II) sulphate to produce potassium sulphate and lead (II) iodide. Reaction type: ___________________________________ Balance the skeletal equation: ______ KI + _____ PbS04 _____K2SO4 + _____PbI2 6. The metal zinc reacts with tin (II) chloride under high heat conditions to produce zinc chloride and tin. Reaction type: ___________________________________ Balance the skeletal equation: ______Zn + _____SnCl2 _____ZnCl2 + _____Sn 7. Sodium hydroxide will be neutralized when combined with hydrochloric acid to produce table salt and water. Reaction type: ___________________________________ Balance the skeletal equation: ______NaOH + _____HCl _____NaCl + _____H2O SNC2D – Chemistry: Chemical Reactions Section 2: Predict the products of the chemical reactions Complete the skeleton equation by predicting the products, balance the equation, and then indicate the type of chemical reaction. 1) __ Ag + __CuSO4 Type:___________________________ 2) __ NaI + __ CaCl2 Type:___________________________ 3) __ Pb(NO3)2 + __ Mn(OH)2 Type:___________________________ 4) __ AgCO3 + __ BaSO4 Type:___________________________ 5) __ Ca(CN)2 + __ CuSO4 Type:___________________________ 6) __ ZnCl2 + __Li Type:___________________________ Table of Polyatomic Ions: (You may find this useful!) Ion Formula NH4+1 ClO3-1 OH-1 NO3-1 MnO4-1 CO3-2 HCO3-1 SO4-2 HSO4-1 PO4-3 CN-1 Ion Name ammonium chlorate hydroxide nitrate permanganate carbonate bicarbonate sulfate bisulfate phosphate cyanide