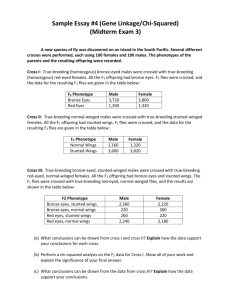
FlyLabNewest - Brandeis University
advertisement

Biology 18a Drosophila Lab Report April 29th, 2008 Phenotypic screen of deficiency libraries implicates gene responsible for regulation of Ptpmeg over-expressed rough eyes in Drosophila Jeremy Gormley Collaborator: S. Gerad Department of Biology, Brandeis University. Supervised by Professor Melissa Kosinski-Collins and Teaching Assistant Yaihara Fortis 2 ABSTRACT In Drosophila, the Ptpmeg gene encodes for a protein responsible for cell signaling and eye development. We determined which gene in Drosophila regulates Ptpmeg expression by performing screens of various deficiency libraries. We crossed virgin female flies over-expressing Ptpmeg with deficiency mutants to look for an enhancement or suppression of the rough-eye phenotype associated with the Ptpmeg virgin flies. A suppressed phenotype was identified and a smaller deficiency mutant was then used in a second cross to determine which gene within the deficiency caused the observed effect. Region 740 was implicated as the region responsible for the suppressed rough eye phenotype and the use of bioinformatics allowed us to localize and characterize possible candidate genes whose deletion caused the suppressed phenotype. Within the deficiency, the Vulcan gene was found to be the enhancer of the Ptpmeg rougheye phenotype. This was determined after relating the nature of Vulcan’s conserved domain to the function of Ptpmeg. Further studies will resolve experimental errors and analyze Vulcan directly to precisely determine how it interacts with Ptpmeg. 2 3 INTRODUCTION Homologs of human genes involved in life-threatening diseases are useful to study when trying to understand the function or regulation of their mammalian counterparts. Experiments that purposely mutate genes to observe the ensuing effects, while not always possible in humans, are uncontroversial when performed with other organisms and may eliminate genes’ potential risks. Drosophila is one organism that shares many genetic and developmental aspects with human beings, thus acting as a useful tool in genetic studies. First, Drosophila is a small species with a short life cycle (approximately 10 days) that can be used to produce a large number of progeny from a small number of parents (Kosinski-Collins, 2008). Second, its genome has been studied extensively and deficiency strains – flies with part of their genomic DNA deleted – are readily available to allow for genetic mapping of small, designated chromosome regions (Beckingham, 2005). Another significant advantage of using drosophila stems from the effectiveness of P elements. P elements are transposons that have been used to analyze gene function in Drosophila in vivo (Beckingham, 2005). A Gal 4 P element, for instance, can express any gene of interest after being inserted into genomic DNA and activating a promoter to control a gene (Beckingham, 2005). Employing Gal4, the Ptpmeg gene was over-expressed in an attempt to determine which gene is responsible for the regulation of Ptpmeg. Ptpmeg is an autosomal gene that encodes for cytoplasmic tyrosine phosphatase proteins which are involved in key cellular events such as passage through the cell cycle and differentiation (Gu, 1996). In particular, Ptpmeg has found to be involved in the eye development of Drosophila and plays an important role in cell-cell signaling that influences neuronal circuit formation in the Drosophila brain (Whited, 2007). In humans, a homolog of Ptpmeg, PTPN3, can act as a colon cancer tumor suppressor gene (Whited, 2007). Thus, analyzing Ptpmeg in Drosophila may help us better understand genes like it in other organisms. In order to determine which gene regulates Ptpmeg, female flies over-expressing wild-type Ptpmeg would be crossed with males of various deficiency strains that cover an enhancer or suppressor of Ptpmeg. Each Ptpmeg construct uses CyO balancers on chromosome 2 while deficiency strains use either CyO balancers or Sb on chromosome 3. The balancers act as dominant markers and prevent recombination between homologous chromosomes. It was expected that the crosses would implicate the Vulcan gene as a regulator of Ptpmeg. Vulcan potentially encodes for guanylate kinase-associate proteins (GKAP) which are known to organize signaling proteins (Romorini, 2004). Vulcan’s influence on neuronal development through signaling implicates it as a regulator of Ptpmeg which, as mentioned, is also involved in cell-cell signaling. If Vulcan’s deletion produced an enhanced rough-eye phenotype, it could be characterized as a suppressor while a suppressed rough-eye phenotype would implicate it as an enhancer. The first cross produced a suppressed rough-eye phenotype which was then observed again in the second cross with a smaller deficiency. Bioinformatics was used to study annotations of the deficiency region within the Drosophila genome, implicating Vulcan as an enhancer of Ptpmeg. 3 4 MATERIALS AND METHODS Experimental crosses with deficiency mutants Drosophila from deficiency strains 1491 and 3003 were separately mated to Ptpmeg virgin flies as described (Kosinski-Collins, 2008). All crosses were incubated at 29°C for two weeks with parent flies transferred to new vials after one week as described (Kosinski-Collins, 2008). F1 progeny were scored as described (Kosinski-Collins, 2008). The process was repeated with the more localized deficiency strains, 3366 and 9503, obtained after class data identified from the first cross which part of the genome was involved in the regulation of the Ptpmeg rough-eye phenotype. Bioinformatics using deficiency strain Based on class data from the second cross, FlyBase was used as described to explore the 740 deletion strain that covered the genomic region h44 - 41A3 in Drosophila (Kosinski-Collins, 2008). Genes deleted by the deficiency were investigated using NCBI’s BLAST as described (Kosinski-Collins, 2008) RESULTS First cross with large deficiency strains After the first cross was set up with large deficiency mutants of the Drosophila genome, the F1 progeny were scored for the presence of an enhanced or suppressed Ptpmeg rough-eye phenotype. Table 1: Observed phenotypes and progeny counts for the F1 progeny of the cross between deficiency strain 3003 (♂) and Ptpmeg virgin flies (♀) Balancer Associated Phenotype Curly Wings, Stubble Bristles Curly Wings Stubble Bristles Eye Phenotype Number of Progeny WT 14 WT 14 Rough 16 Straight wings, Long Bristles Rough 20 WT refers to wild-type eye and Rough refers to rough eye similar to the Ptpmeg virgin females. The phenotypes of the flies’ wings, bristles, and eyes were determined by inspection for the cross with deficiency 3003. As shown in Table 1, 14 flies were observed with curly wings, stubble bristles and wild-type eyes; 14 flies were observed with curly wings and wild-type eyes; 16 flies were observed with stubble bristles and rough eyes; and 20 flies were observed with straight wings, long bristles, and rough eyes. Table 2: Observed phenotypes and progeny counts for the F1 progeny of the cross between deficiency strain 1547 (♂) and Ptpmeg virgin flies (♀) Balancer Associated Phenotype Curly Wings Curly Wings Eye Phenotype Number of Progeny - - WT 21 Rough 23 Straight wings, Long Bristles Rough 27 4 5 WT refers to wild-type eye and Rough refers to rough eye similar to the Ptpmeg virgin females The phenotypes of the flies’ wings, bristles, and eyes were determined by inspection for the cross with deficiency 1547. As shown in Table 2, 21 flies were observed with curly wings and wildtype eyes; 23 flies were observed with curly wings and rough eyes; and 27 flies were observed with straight wings, long bristles, and rough eyes. Table 3: Observed phenotypes and progeny counts for the F1 progeny of the cross between deficiency strain 1491 (♂) and Ptpmeg virgin flies (♀) Balancer Associated Phenotype Curly Wings Curly Wings Eye Phenotype Number of Progeny - - WT 22 Rough 22 Straight wings, Long Bristles Rough 40 WT refers to wild-type eye and Rough refers to rough eye similar to the Ptpmeg virgin females The phenotypes of the flies’ wings, bristles, and eyes were determined by inspection for the cross with deficiency 1491. As shown in Table 3, 22 flies were observed with curly wings and wildtype eyes; 22 flies were observed with curly wings and rough eyes; and 40 flies were observed with straight wings, long bristles, and rough eyes. Table 4: Observed phenotypes and progeny counts for the F1 progeny of the cross between deficiency strain 3007 (♂) and Ptpmeg virgin flies (♀) Balancer Associated Phenotype Curly Wings, Stubble Bristles Curly Wings Stubble Bristles Eye Phenotype Number of Progeny WT 11 WT 11 Rough 11 Straight wings, Long Bristles Rough 17 WT refers to wild-type eye and Rough refers to rough eye similar to the Ptpmeg virgin females. The phenotypes of the flies’ wings, bristles, and eyes were determined by inspection for the cross with deficiency 3007. As shown in Table 4, 11 flies were observed with curly wings, stubble bristles and wild-type eyes; 11 flies were observed with curly wings and wild-type eyes; 11 flies were observed with stubble bristles and rough eyes; and 17 flies were observed with straight wings, long bristles, and rough eyes. A class-wide analysis was carried out on F1 progeny from various deficiency strains. As shown in tables 1 – 4, our individual crosses did not produce an enhanced or suppressed Ptpmeg rougheye phenotype. However, a suppressed Ptpmeg rough eye phenotype was observed for the cross with the 739 deficiency strain. 5 6 Second cross with smaller deficiency strains A second cross was set up with smaller deficiency strains to localize the region within the deletion of the 739 strain responsible for the observed phenotype. Table 5: Observed phenotypes and progeny counts for cross between deficiency strain 9503 (♂) and Ptpmeg/Cy (♀) Balancer Associated Phenotype Curly Wings Curly Wings Eye Phenotype Number of Progeny - - WT 19 Rough 52 Straight wings, Long Bristles Rough 27 WT refers to wild-type eye and Rough refers to rough eye similar to the Ptpmeg virgin females. The phenotypes of the flies’ wings, bristles, and eyes were determined by inspection for the cross with deficiency 9503. As shown in Table 5, 19 flies were observed with curly wings and wildtype eyes; 52 flies were observed with curly wings and rough eyes; and 27 flies were observed with straight wings, long bristles, and rough eyes. Table 6: Observed phenotypes and progeny counts for cross between deficiency strain 3366 (♂) and Ptpmeg/Cy (♀) Balancer Associated Phenotype Curly Wings, Stubble Bristles Curly Wings Stubble Bristles Eye Phenotype Number of Progeny WT 9 WT 17 Rough 8 Straight wings, Long Bristles Rough 6 WT refers to wild-type eye and Rough refers to rough eye similar to the Ptpmeg virgin females. The phenotypes of the flies’ wings, bristles, and eyes were determined by inspection for the cross with deficiency 3366. As shown in Table 6, 9 flies were observed with curly wings, stubble bristles and wild-type eyes; 17 flies were observed with curly wings and wild-type eyes; 8 flies were observed with stubble bristles and rough eyes; and 6 flies were observed with straight wings, long bristles, and rough eyes. A class-wide analysis was carried out on F1 progeny from the second. Once again, as shown in table 5 and 6, our individual results did not produce a suppressed Ptpmeg rough-eye phenotype. After gathering class data, a suppressed Ptpmeg rough eye phenotype was found in the cross with the 740 deficiency strain. 6 7 Observed eye phenotype of Drosophila The different eye phenotypes observed were photographed for comparison. Figure 1: Ptpmeg Rough-Eye Phenotype in Drosophila Drosophila exhibiting wild-type eyes (left) compared to Drosophila with Ptpmeg rough eyes Figure 2: Suppression of Ptpmeg Rough-eye Phenotype in Drosophila Drosophila with Ptpmeg rough eyes (left) compared to Drosophila with suppressed Ptpmeg rough eyes As shown in Figure 1, the Ptpmeg rough eye phenotype is darker than the wild type eyes with less defined ommatidium. The suppressed Ptpmeg rough-eye phenotype, as shown in Figure 2, is lighter and resembles the wild type eyes. 7 8 Bioinformatics After identifying the 740 deficiency strain as the one associated with the suppressed Ptpmeg rough eye phenotypes, the deleted region was analyzed using FlyLab. The 740 deficiency strain, Df(2R)M41A8, was found to cover the h44 – 41A3 region of the genome on the right arm of chromosome 2. The common genes in the 739 and 740 deficiency strains were analyzed and their functions, if known, were determined. Table 5: Gene Information deleted by deficiency strain Df(2R)M41A8 Number of Gene Name of Gene Function Gene 1 vlc (Vulcan) Gene 2 Nipped-A unknown transcription regulatory activity, protein kinase activity Gene 3 Nipped-B transcription regulatory activity Gene 4 p120 ctn binding, signal transduction Four common genes (Vulcan, Nipped-A, Nipped-B, and p120 ctn) were found in the overlapping regions between strains 739 and 740. Vulcan, as shown in Table 5, was the only gene found to have an unknown function and was thus characterized using NCBI’s BLAST sequencing program. Table 6: Conserved Domains of unknown genes of Df(2R)M41A8 Number of Gene Name of Gene Gene 1 vlc (Vulcan) Conserved Domain guanylate kinase-associated protein (GKAP) Vulcan was characterized by the presence of a conserved domain. As shown in Table 6, a conserved domain associated with guanylate kinase-associate protein (GKAP) was detected. DISCUSSION When the F1 progeny from the crosses were scored, flies with straight wings and long bristles corresponded to the Ptpmeg over Deficiency (Ptpmeg/Def) genotype since the phenotypes associated with Sb and CyO balancers were absent. These progeny were of interest to observe the effect of the Deficiency on the Ptpmeg rough eyes. As Tables 1-4 demonstrate, 20 F1 Ptpmeg/Def progeny were observed for the cross with deficiency 3003; 27 for the cross with 1547; 40 for the cross with 1491; and 17 for the cross with 3007. All of these F1 progeny with straight wings and straight bristles showed no enhancer or suppressor but only the same rough eyes that the Ptpmeg virgin female flies exhibited. Class data showed that crosses with deficiency strain 739 revealed a suppressed phenotype. This implicated an enhancer within the deleted region that regulates Ptpmeg’s signaling activity involved in eye development. F1 progeny from the second cross with deficiency strains 3366 and 9503 were analyzed for the same suppressed Ptpmeg rough-eye phenotype since the strains used in the second cross covered a smaller region of the Drosophila genome overlapping the implicated 739 strain. As Tables 5 and 6 demonstrate, 27 F1 Ptpmeg/Def progeny were observed for the cross with deficiency 9503 and 6 for the cross with 3366. Once again, however, these flies with straight wings and long bristles did not produce a suppressed Ptpmeg rough-eye phenotype. Class data, however, 8 9 implicated deficiency strain 740 since its cross with Ptpmeg flies resulted in suppressed rougheye phenotype for the Ptpmeg/Def progeny. For both the first and second set of crosses, a 1:4 ratio was expected for the F1 progeny from crosses with deficiency strains 3003, 3007, and 3366 which had Sb balancers. Only a 1:3 ratio was expected for the 1547, 1491, and 9503 strain which had CyO balancers, however, since the progeny with CyO/Cyo genotypes would die due to homozygous balancers. Yet, the progeny count did not conform to these ratios in any cross, suggesting that the deficiencies likely fatally harmed the development of the flies. If the experiment were to be changed to include less harmful balancers, the counts may have more accurately represented the expected genotypic ratios. Experimental errors might have also influenced the progeny count. Incubation conditions may not have allowed for proper fly development. An incubation temperature set too high, for example, may have made female flies sterile, affecting the progeny count. It is also still possible that some female flies from the deficiency strains erroneously entered the vials. Through Bioinformatics, the Vulcan gene was identified as the enhancer of Ptpmeg due to the suppression of the Ptpmeg rough-eye phenotype. Vulcan was expected to regulate protein function level rather than gene level expression since the Ptpmeg constructs over-expressed Ptpmeg with a recombinant promoter, introduced with P elements. After analyzing Vulcan’s conserved domain, as shown in Table 6, it is evident that, since Vulcan may encode for guanylate kinase-associated proteins (GKAPs), it indeed may potentially influence Ptpmeg via proteinprotein binding domains which affect protein expression. Furthermore, since GKAPs are known to organize signaling proteins, Vulcan may be involved in the same neuronal transmissions necessary for proper eye formation as Ptpmeg. Thus, without Vulcan, Ptpmeg did not have the full ability to control cell-cell signaling pathways and axonal activity to maintain the Ptpmeg rough-eye phenotype. Furthermore, the findings support the hypothesis that an observed suppressed rough-eye phenotype implicates Vulcan as an enhancer and, while it is possible that other factors may have also contributed to a reduction in the rough-eye phenotype, the experiment verified Vulcan’s connection to Ptpmeg. While Vulcan was identified as the enhancer present in the deleted region of deficiency 740, Vulcan’s gene function remains known. This lends to the need for a further examination of the regulation of Ptpmeg. Future experiments may include studying the effects that Vulcan’s deletion in the genome has on neuronal development of the fly, in addition to determining whether the genes of known function within deficiency 740 may also regulate Ptpmeg in some way that compliments Vulcan’s function. REFERENCES: Kosinski-Collins, M. Spring 2008. Biology 18a Laboratory Manual. Brandeis University, Waltham, MA. Beckingham, K. M., Armstrong, J. D., Texada, M. J., Munjaal, R., and Baker, D. A. 2005. Drosophila Melanogaster – The Model Organism of Choice for the Complex Biology of Multi-cellular Organisms. Gravitational and Space Biology 18: 17 – 30. Gu, M., and Majerus, P. W. 1996. The Properties of the Protein Tyrosine Phosphatase PTPMEG. The Journal of Biological Chemistry 271: 27751–27759 9 10 Whited, J. L., Robichaux, M. B., Yang, J. C., and Garrity, P. A. 2007. Ptpmeg is required for the proper establishment and maintenance of axon projections in the central brain of Drosophila. Development 134: 43 - 53 Romorini, S., Piccoli, G., Jiang, M., Grossano, P., Tonna, N., Passafaro, M., Zhang, M., and Sala, C. 2004. A Functional Role of Postsynaptic Density-95-Guanylate KinasAssociate Protein Complex in Regulating Shank Assembly and Stability to Synapses. The Journal of Neuroscience. 24: 9391 - 9404 10