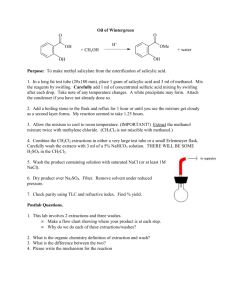
Oxidation of Methanol using copper as a catalyst
advertisement
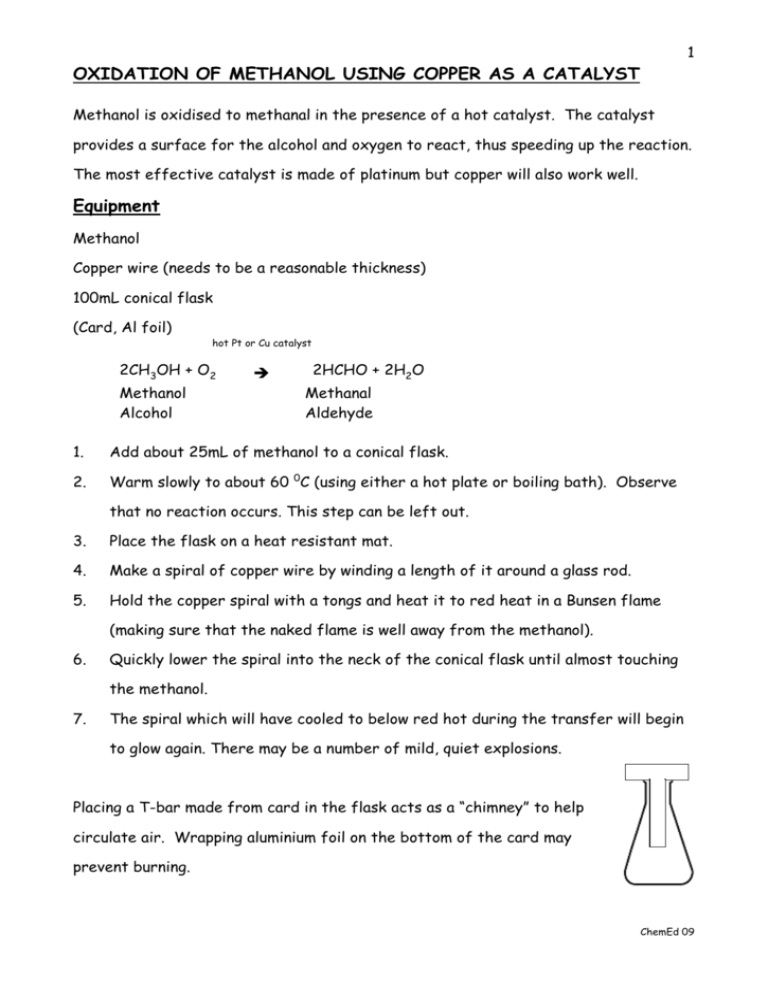
1 OXIDATION OF METHANOL USING COPPER AS A CATALYST Methanol is oxidised to methanal in the presence of a hot catalyst. The catalyst provides a surface for the alcohol and oxygen to react, thus speeding up the reaction. The most effective catalyst is made of platinum but copper will also work well. Equipment Methanol Copper wire (needs to be a reasonable thickness) 100mL conical flask (Card, Al foil) hot Pt or Cu catalyst 2CH3OH + O2 Methanol Alcohol 2HCHO + 2H2O Methanal Aldehyde 1. Add about 25mL of methanol to a conical flask. 2. Warm slowly to about 60 0C (using either a hot plate or boiling bath). Observe that no reaction occurs. This step can be left out. 3. Place the flask on a heat resistant mat. 4. Make a spiral of copper wire by winding a length of it around a glass rod. 5. Hold the copper spiral with a tongs and heat it to red heat in a Bunsen flame (making sure that the naked flame is well away from the methanol). 6. Quickly lower the spiral into the neck of the conical flask until almost touching the methanol. 7. The spiral which will have cooled to below red hot during the transfer will begin to glow again. There may be a number of mild, quiet explosions. Placing a T-bar made from card in the flask acts as a “chimney” to help circulate air. Wrapping aluminium foil on the bottom of the card may prevent burning. ChemEd 09