Mesure de OH et HO2 par techniques
advertisement

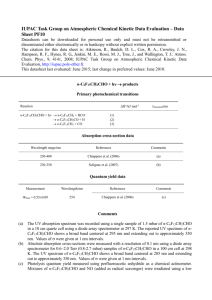
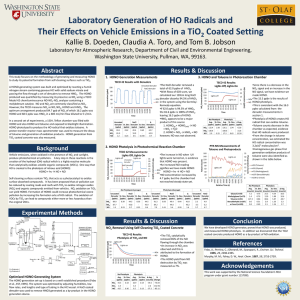
Spectroscopic study of HONO using laser photolysis coupled to high repetition rate LIF and cw-CRDS C. Jain, P. Morajkar, C. Schoemaecker et C. Fittschen Laboratoire PC2A, Université de Lille 1- Bâtiment C11, 59655 Villeneuve d’Ascq, France * christa.fittschen@univ-lille1.fr 1 Nitrous acid (HONO) is an important chemical species in the atmosphere as well as in laboratory studies. In the atmosphere it is, especially in polluted areas, a major photochemical precursor for OH radicals in the early morning, but has also been detected in remote areas such as the south pole. HONO can be produced in a simple gas phase reaction between OH radicals and NO, but heterogeneous reactions seem to be more important and heterogeneous formation has been shown to occur on ice, but also on photocatalytic surfaces. HONO can also be an interesting OH-precursor for laser photolysis studies, because it generates OH radicals after 351 nm excimer photolysis, thus avoiding possible unwanted complications arising at shorter wavelengths such as 248nm. HONO is often detected and quantified by spectroscopic methods: UV-VIS absorption using open path DOAS technique has been very successful for atmospheric measurements [1], but FTIR spectroscopy has also been used, especially in laboratory studies [2]. While the qualitative absorption spectra of HONO have been studied by many authors in the UV-VIS and IR range, the major difficulty using spectroscopic detection methods is the uncertainty linked to the absolute absorption cross sections: HONO exists in an equilibrium with other components such as NO, NO2, HNO3 and H2O, and is a rather unstable molecule, decomposing easily through heterogeneous reactions. Therefore, determining the absolute HONO concentration contained in an absorption cell is rather difficult. Febo et.al. [3] have developed a method allowing the generation of stable HONO flows with very high purity, and this method has subsequently been used to determine absorption cross sections in the UV [4] and in the IR [5]. Cavity enhanced absorption spectroscopy is getting more and more popular, especially in the near IR range, where cheap and reliable DFB lasers and detectors are available. This makes this spectroscopic range attractive, because now the rather small absorption cross sections expected for overtone transitions occurring in this wavelength range can be compensated for by the high sensitivity of the cavity enhanced methods. HONO is known to exist in an equilibrium in two different forms, trans- and cis-HONO in a ratio of approximately 2 to 1 in favour of the trans form [6]. The rovibrational parameters of both isomers, including the 21 overtone of the OH stretch, have been published [7, 8]: the band centre of the 21 overtone of the cis-isomer has been located at around 6665 cm-1, close to well-known and relatively intense 21 overtone features of the HO2 radical, an important intermediate in the oxidation of VOCs. The knowledge of absolute absorption cross sections for some HONO absorption lines in this wavelength range would allow for future, simultaneous measurements of HONO and HO2 in laboratory studies. Only one fairly indirect measurement of absorption cross sections for HONO is available in this range: Djehiche et.al. [9] have detected HONO by cw-CRDS at 6625.69 cm-1 as reaction product during the photolysis of CH3ONO in a simulation chamber. Through simultaneous measurements of CH 2O and subsequent modelling they deduced the theoretical HONO concentration, postulating that it is formed in a reaction of OH radicals with the precursor CH3ONO. From this fairly indirect approach they have estimated the absorption cross section for cis-HONO to 6625.69cm-1 = 4.2 10-21 cm2, assuming that only 1/3 of the formed HONO is in cis-configuration. We present here the measurement of the HONO spectrum in the range 6623.6 - 6645.6 cm-1 using two different, complementary methods for generating HONO: pulsed and continuous. The pulsed method has allowed determining absolute absorption cross sections, while the continuous method led to a better signal-tonoise ratio. While the absorption cross sections are probably too small for a future quantification of HONO in the real atmosphere, these results will allow quantitative determination of HONO in laboratory and chamber experiments. Références [1] D. Perner, U. Platt, Geophysical Research Letters 6, 917. [2] R. S. Karlsson, E. B. Ljungstrom, Environmental Science & Technology 30, 2008 (1996). [3] A. Febo, C. Perrino, M. Gherardi, R. Sparapani, Environmental Science & Technology 29, 2390 (1995). [4] J. Stutz et al., J. Geophys. Res. 105, 14585 (2000). [5] W. S. Barney et al., The Journal of Physical Chemistry A 104, 1692 (2000). [6] R. Varma, R. F. Curl, The Journal of Physical Chemistry 80, 402 (1976). [7] Guilmot J. M., Godefroid M., Herman M., Journal of Molecular Spectroscopy 160, 387 (1993). [8] Guilmot J. M., Melen F., Herman M., Journal of Molecular Spectroscopy 160, 401 (1993). [9] M. Djehiche, A. Tomas, C. Fittschen, P. Coddeville, Environmental Science & Technology 45, 608 (2011).