Abstract
advertisement

Abstract
Abstract
The thesis entitled “Synthesis of (-) Cladospolide-B, Studies Towards iso-
Cladospolide-B and Cladospolide-C; Synthesis of C-Linked Deoxy Spiro
Disaccharides and Some Useful Synthetic Transformations” is divided into
three chapters.
Chapter I: Stereoselective total synthesis of (-) Cladospolide-B, studies
directed towards the total synthesis of iso-Cladospolide-B and
Cladospolide-C
This chapter deals with the stereoselective total synthesis of (-) cladospolide-B and
attempted synthesis of iso-cladospolide-B from L (+)-tartaric acid and cladopsolide-C
from D (+)-glucose.
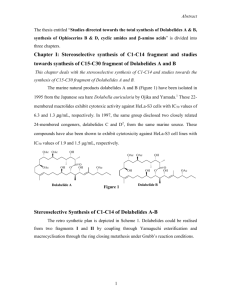
Cladospolide-B (1) (Figure 1), novel 12-member macrolide, is a metabolite
produced by fungus Cladosporium cladosporioides FI-113,1 Cladosporium tenuissimum2
and marine fungal species,3 showed plant growth retardant activity to rice seedlings,
while cladospolide-C (3) inhibits root growth of lettuce seedlings.2 Compound 1 also acts
as inhibitor of allergy and inflammation.4 A stereoselective total synthesis of ()cladospolide-B (1) and studies towards the synthesis of iso-cladospolide-B (2) and
cladospolide-C (3) are described.
Figure 1
OH
OH
O
O
O
OH
O
OH
1
2
OH
O
OH
O
OH
O
OH
O
3
4
1
Abstract
Stereoselective total synthesis of (-) cladospolide-B (1), studies directed
towards the total synthesis of iso-cladospolide-B (2)
The retrosynthetic plan for 1 and 2 are depicted in Scheme 1. The seco-acid 5 on
esterification under Yamaguchi5 reaction conditions would give 1 and 2, while the secoacid 5 could be a late stage intermediate. Acid 5, in turn, could be prepared from 6, while
6 would be prepared from 7. In turn, 7 could be prepared from L (+)-tartaric acid and 1,5pentane diol. Thus, the basic strategy for the synthesis of the key fragment 7 was to
utilize the pair of stereocenters in tartaric acid as C-4 and 5 in target molecule and to
introduce the C-11 stereocentre by Jacobsen6 reaction.
Scheme 1
O
OH
+ O
O
OH
OH
O
O
OH
2
1
OH
OH
5
O
O
OH
O
O
O
OAc
OTPS
OBn
O
6
7
H
O
O
OBn
O
L (+)-tartaric acid
8
+
-
IPh3P+
OTPS
1,5-pentane diol
9
Accordingly, the known diester 10 (Scheme 2), derived from L (+)-tartaric acid,
was treated with 2,2-DMP in dry DMSO in acidic medium to give 11, which on reduction
with LAH in dry THF gave the diol 12. Selective benzylation of 12 with BnBr and
K2CO3 in refluxing acetone furnished 13, while 13 on Swern7 oxidation gave aldehyde 8.
The salt 9 was prepared from 1,5-pentane diol. Accordingly, 1,5-pentane diol on
treatment with TBDPSCl and imidazole in CH2Cl2 furnished 14, which on reaction with
iodine, Ph3P and imidazole in toluene under reflux furnisged 15. Iodo compound 15 on
2
Abstract
reaction with Ph3P in toluene reflux furnished 9. Salt 9 was treated with n-BuLi to form
ylide, which on reaction with aldehyde 8 furnished 7.
Scheme 2
O
a
L (+)-Tartaric acid
O
HO
O
b
OMe
OMe
O
HO
c
OMe
OMe
O
10
d
O
OH
OR
O
O
12 R = H
13 R = Bn
11
O
O
e
H
OBn
O
8
1,5-pentane diol
f
HO
g
OTPS
I
OTPS
14
h
15
O
I-H3PP+
OTPS
O
H
OBn
+
O
9
8
i
O
OTPS
OBn
O
7
Reagents and conditions: a) MeOH/H+, reflux, 12 h, 88%; b) 2,2-DMP, H+, dry DMSO, rt, 6 h,
89%; c) LAH, dry THF, 0 oC, 4 h, 92%; d) K2CO3, BnBr, acetone, reflux, 12 h, 77%; e) (COCl)2,
dry DMSO, Et3N, CH2Cl2, -78 oC, 2 h, 91%; f) TBDPSCl, imidazole, CH2Cl2, 0 oC, 12 h, 76%;
g) I2, Ph3P, imidazole, toluene, rt, 30 min, 82%; h) Ph3P, toluene, reflux, 24 h, 82%; i) n-BuLi,
dry THF, 0 oC, 2 h, 75%.
Deprotection of the TBDPS ether in 7 with TBAF and subsequent oxidation of 16
under Swern reaction conditions furnished aldehyde 17, which on reaction with TMSOI
and t-BuOK in dry DMSO afforded recaemic epoxide 18 (Scheme 3). The chiral epoxide
19 was obtained from 18, which on Jacobsen resolution with S,S-Jacobsen catalyst
affforded S-epoxide. Epoxide 19 on reduction with LAH furnished 21, which on
acetylation with AcCl and Et3N in CH2Cl2 in the presence of DMAP gave 22. Reaction of
22 with Pd/C in EtOAc under hydrogen gas gave 6, which on oxidation with IBX in dry
DMSO furnished aldehyde 23. Reaction of 23 with (methoxycarbonylmethylene)
triphenyl phosphorane gave 24 and 25.
3
Abstract
Scheme 3
a, b
7
R c
O
O
OBn
O
18
OH
O
OH
O
+
OBn
O
d
OBn
O
16 R = CH2OH
17 R = CHO
O
O
OBn
O
19
20
OR
19
e, f
O
g, b
O
OBn
O
O
O
O
O
O
OAc
6 R = CH2OH
23 R = CHO
21 R = H
22 R = Ac
h
R
OMe
OAc
+
OMe OAc
O
O
24
25
Reagents and conditions: a) TBAF, dry CH2Cl2, 12 h, 93%; b) (COCl)2, dry DMSO, Et3N, CH2Cl2, -78
o
C, 2 h, 90%; c) TMSOI, t-BuOK, dry DMSO, 0 oC, 1 h, 53%; d) S,S-Jacobsen catalyst, Et3N, rt, 12 h,
40%; e) LAH, dry THF, 0 oC, 1 h, 89%; f) AcCl, Et3N, DMAP, CH2Cl2, 0 oC, 2 h, 89%; g) H2, Pd/C,
EtOAc, rt, 12 h, 89%; h) Ph3P=CHCO2Me, MeOH, 0 oC, 2 h, 87%.
Ester 24 was hydrolyzed with 4N NaOH in MeOH to give seco acid 5, which on
macrolactonisation under Yamaguchi reaction conditions (2,4,6-trichlorobenzoyl
chloride, Et3N, THF, DMAP, toluene) afforded 26. Finally, deprotection of
isopropylidene group in 26 was effected with 80% aq. AcOH at 70 oC to give 1 and 2 as
solids. The 1H and
13
C NMR data and melting point of synthetic 1 were in good
accordance with that of the natural product but optical rotation value of synthetic 1 was
not matching with the natural one. However, this value is compared with the data of (-)
cladospolide-B recently synthesized by Banwell et al.8 The 1H and
13
C NMR data,
melting point and optical rotation values of synthetic 2 was not matching with the natural
product and moreover the absolute chemistry of iso-cladospolide-B was not defined in
literature.
4
Abstract
Scheme 4
O
a
24
O
O
b
O
OH
O
O
OH
O
26
5
OH
OH
O
c
O
+
OH
O
O
OH
1
2
Reagents and conditions: a) 4N NaOH, MeOH, rt, 4 h, 84%; b) 2,4,6-trichlorobenzoyl chloride, Et3N,
CH2Cl2, DMAP, toluene, reflux, 24 h, 75%; c) 80% aq. AcOH, 70 oC, 1 h, 70%.
Studies directed towards the total synthesis of Cladospolide-C
Cladospolide-C (3), another novel 12-membered macrolide, is a fungal metabolite
of soil fungi, Cladosporium tenuissimum2 and inhibited root growth of lettuce seedlings.
A formal synthesis of cladospolide-C (3) is described.
The retrosynthetic plan for 3 (Scheme 2) indicates, the seco acid 27 as a late stage
intermediate, while 27 in turn, could be prepared from 28. Compound 28 was envisaged
from 29, which inturn, could be obtained from 30. Finally, 30 in turn, could be made
from ‘diacetone glucose’ 31. Thus, the basic strategy for the synthesis of the key
fragment 30 is to utilize C-3 and 4 in ‘diacetone glucose’ as C-4 and 5 in the target
molecule and introduced C-11 stereocentre by Jacobsen hydrolytic kinetic resolution
reaction.
Scheme 5
OBn
O
OH
O
OH
OH
OH
OH
O
OBz
BnO
O
O
O
3
28
27
O
TPSO
O
O
BnO
O
O
O
BnO
O
30
29
5
O
O
O
HO
O
O
31
Abstract
Accordingly, D (+)-glucose was treated with acidic acetone at room temperature
to afford ‘diacetone glucose’ 31 (Scheme 6), which on reaction with NaH and BnBr in
DMF afforded 32. Reaction of 32 with 60% aq. AcOH furnished diol 33, which on
oxidative cleavage (H5IO6, EtOAc:H2O, 0 oC) furnished aldehyde 34. The Wittig
olefination on 34, with 9 in the presence of n-BuLi in dry THF furnished 30, which on
partial hydrogenation with catalytic PtO2 gave 35. Deprotection of TBDPS ether in 35
with TBAF and subsequent oxidation of 36 with IBX furnished aldehyde 37, which on
treatment with TMSOI and t-BuOK in dry DMSO gave racaemic epoxide 29. The
mixture of epoxides were resolved under Jacobsen hydrolytic kinetic resolution reaction
(S,S-Jacobsen catalyst, H2O, RT) to furnish S-epoxide 38 and R-diol 39.
Scheme 6
O
O
O
a, b
D (+)-Glucose
HO
RO
O
O
H
BnO
O
c
O
O
31 R = H
32 R = Bn
d
O
BnO
O
33
TPSO
e
O
BnO
O
f, g, h
O
O
30
34
O
R
O
HO
O
i
O
O
BnO
O
35 = CH2OTPS
36 = CH2OH
37 = CHO
O
O
BnO
O
BnO
29
O
j
O
+
O
O
HO
BnO
38
O
OH
O
39
+
Reagents and conditions: a) Acetone/H , rt, 12 h, 78%; b) NaH, BnBr, DMF, 0 oC, 12 h, 78%; c)
60% aq. AcOH, rt, 12 h, 90%; d) H5IO6, EtOAc:H2O, 0 oC, 1 h, 89%; e) 9, n-BuLi, dry THF, -78 oC,
2 h, 74%; f) H2, PtO2, EtOAc, rt, 12 h, 93%; g) TBAF, dry CH2Cl2, rt, 12 h, 90%; h) {COCl)2, dry
DMSO, Et3N, CH2Cl2, -78 oC, 2 h, 95%; i) TMSOI, t-BuOK, dry DMSO, 0 oC, 1 h, 78%; j)
S,S-Jacobsen catalyst, toluene, H2O, rt, 12 h, 43%.
The chiral S-epoxide 38 was reduced with LAH to furnish 40, which on
benzoylation with BzCl, Et3N and DMAP in CH2Cl2 furnished 28 (Scheme 7). The 1,2-
6
Abstract
O-isopropylidene group in 28 was hydrolyzed with 60% aq. AcOH at 70 oC to furnish
lactol 41, which on oxidative cleavage with H5IO6 furnished aldehyde 42. The aldehyde
42 reacted with (methoxycarbonylmethylene)triphenyl phosphorane to furnish E-olefin
43, which on hydrolysis with 4N NaOH in methanol furnished seco-acid 27. The secoacid on macrolactonization under Yamaguchi reaction conditions (2,4,6-trichlorobenzoyl
chloride, Et3N, THF, DMAP, toluene reflux) furnished 44. Finally, deprotection of the
benzyl group in 44 was effected with TiCl4 in CH2Cl2 at 0 oC to furnish 3 as a white
solid. The 1H NMR, m. p. and optical rotation values of synthetic 3 were not in
accordance with that of the natural product.
Scheme 7
O
a, b
38
O
c
O
BnO
BnO
O
40 R = H
28 R = Bz
OBn
e
CHO
f
OMe
HCOO
OBz
HCOO
O
43
42
OBn
OH
OH
OH
41
OBn
OBz
d
OH
OBz
OR
OH
OH
g
O
O
OH
OBn
O
O
O
27
OH
h
44
3
Reagents and conditions: a) LAH, dry THF, 0 oC, 1 h, 81%; b) BzCl, Et3N, DMAP, CH2Cl2, 0 oC, 2
h, 95%; c) 60% aq. AcOH, 70 oC, 12 h, 85%; d) H5IO6, EtOAc:H2O, 0 oC, 1 h, 90%; e)
Ph3P=CHCO2Me, toluene, reflux, 2 h, 89%; f) 4N NaOH, CH3OH, rt, 2 h, 72%; g)
2,4,6-trichlorobenzoyl chloriide, Et3N, THF, DMAP, toluene, reflux, 24 h, 66%; h) TiCl4, dry CH2Cl2,
0 oC, 2 h, 74%.
Spectral data of the synthesized molecule 3 did not match with the reported data
of cladospolide-C and moreover the absolute chemistry of cladospolide-C not defined in
literature. Hence, we undertook the synthesis of another diastereomer based on
assumption starting from the diol 39 obtained during the Jacobsen hydrolytic kinetic
resolution. Compound 4 (C-11 epimer of 3) was synthesized by the same strategy used
for compound 3 (Scheme 8).
7
Abstract
Scheme 8
OBn
O
OH
O
OH
OBz
O
OH
OH
OH
BnO
O
O
O
4
45
46
O
HO
O
OH
BnO
O
39
Accordingly, the diol 39, was tosylated (TsCl, Et3N, CH2Cl2) to furnish 47
(Scheme 9), which on deoxygenation with LAH in dry THF furnished 48. The free –OH
Scheme 9
a
39
O
TsO
O
b, c
O
O
OR
OH
BnO
BnO
O
47
O
48 R = H
46 R = Bz
OBn
O
d
e
OH
CHO
OBz
BnO
OBz
OH
HCOO
49
50
OBn
OBn
OMe
OBz
f
HCOO
51
g
OH
OH
O
OH
O
45
OH
h
O
OH
i
O
OBn
O
OH
O
52
4
o
Reagents and conditions: a) p-TsCl, Et3N, CH2Cl2, 0 C, 12 h, 71%; b) LAH, dry THF, 0
o
C, 1 h, 75%; c) BzCl, Et3N, DMAP, CH2Cl2, 0 oC, 2 h, 78%; d) 60% aq. AcOH, 70 oC, 12
h, 82%; e) H5IO6, EtOAc:H2O, 0 oC, 1 h, 87%; f) Ph3P=CHCO2Me, toluene, reflux, 2 h,
88%; g) 4N NaOH, CH3OH, rt, 2 h, 81%; h) 2,4,6-trichlorobenzoyl chloriide, Et3N, THF,
DMAP, toluene, reflux, 24 h, 79%; i) TiCl4, dry CH2Cl2, 0 oC, 2 h, 74 %.
8
Abstract
in 48 was protected with benzoyl group (BzCl, Et3N, DMAP, CH2Cl2) to furnish 46. The
1,2-O-isopropylidene group in 46 was hydrolyzed with 60% aq. AcOH at 70 oC to furnish
lactol 49, which on oxidative cleavage with H5IO6 furnished aldehyde 50. Reaction of 50
with (methoxycarbonylmethylene)triphenyl phosphorane furnished 51, which on
hydrolysis with 4N NaOH in methanol gave acid 45. The seco acid 45 on
macrolactonization under Yamaguchi reaction conditions furnished 52. Finally,
deprotection of the benzyl group in 52 was effected with TiCl4 in CH2Cl2 at 0 oC to
furnish 4 as a white solid. The 1H NMR, m. p. and optical rotation values of synthetic 4
also were not in accordance with that reported for the natural product.
Chapter II: Stereoselective synthesis of
C-linked deoxy spiro
disaccharides
This Chapter deals with the synthesis of C-linked 2-deoxy and 4-deoxy spiro
disaccharides. Homopropargyl and allyl moieties introduced at the C-3 of xylofuranoside
were converted into the deoxy sugar moieties.
Stereoselective synthesis of C-linked 2-deoxy spiro disaccharides
Though several synthesis of glycosyl mimics, such as C-glycosides, C-saccharides
etc., are reported, much work has not been done on the synthesis of spiro Cdisaccharides, in which the sugars are attached through a ‘spiro’ carbon atom. Recently
we have demonstrated the use of furan for the first synthesis of spiro carbon linked
disaccharides.9 Our continued interest on the use of carbohydrate derived chiral templates
for the synthesis of various bio-active compounds as well as new glycosubstances,
prompted us to synthesize the ‘spiro’ carbon linked deoxy disaccharides 1, 2, 3 and 4.10
The newly constructed sugar moiety since is a deoxy sugar; deoxy disaccharides 1-4 are
named as ‘spiro’ carbon linked 2-deoxy disaccharides (Figure 1).
9
Abstract
In the present study, the main strategy is to add a 4-carbon synthon,
homopropargylic alcohol unit, onto the ‘chirons’ derived from ‘xylose’, wherein,
chirality is imparted from the sugar chiron, while the homopropargylic moiety would be
manipulated into a sugar moiety (Scheme 1). Accordingly, xylose on reaction with
acetone and aq. Na2CO3 in acidic conditions furnished diol 5, which on reaction with
TBDMSCl and imidazole in CH2Cl2 furnished 6. Oxidation of 2o hydroxyl of 9 with
PDC, MS 4Ǻ and Ac2O in CH2Cl2 at reflux furnished 7. Compound 7 on reaction with
PMB protected homopropargyl alcohol and n-BuLi in THF furnished 8. Triple bond in 8
was reduced with LAH to afford E-olefin, wherein, deprotection of TBDMS also was
observed. The free –OH group in 9 was protected again with TBDMS (TBDMSCl and
imidazole in CH2Cl2) to furnish 10. Compound 10 was subjected to osmylation11 with
OsO4 and NMO to furnish 11 and 12, both of which were acetylated with Ac2O and Et3N
Scheme 1
D (+)-Xylose a
HO
O
O
O
HO
PMBO
O
e
O
PMBO
OH
10
O
O
OH
9
O
OH OH
11
O
h
O
O
7
O
O
OH
8
O
O
PMBO
TBDMSO
HO
PMBO
TBDMSO
6
O
c
O
HO
O
d
O
HO
5
TBDMSO
TBDMSO
b TBDMSO
O
f
O
TBDMSO
AcO
PMBO
O
OAc OH
13
O
O
g
O
TBDMSO
HO
PMBO
O
OH OH
12
O
O
h
TBDMSO
AcO
PMBO
O
O
OAc OAc O
14
Reagents and conditions: a) H+, acetone, aq.Na2CO3, < 20 oC, 6 h, 80%; b) TBDMSCl, imidazole,
CH2Cl2, 0 oC, 6 h, 70%; c) PDC, MS 4 Ao, Ac2O, CH2Cl2, reflux, 12 h, 52%; d) HCCCH2CH2OPMB,
n-BuLi, dry THF, -78 oC, 2 h, 50%; e) LAH, THF, 0 oC, 4 h, 77%; f) TBDMSCl, imidazole, CH2Cl2, 0
o
C, 12 h, 80%; g) OsO4, NMO, acetone:water (3:1), rt, 6 h, 80%; h) Ac2O, Et3N, CH2Cl2, 0 oC, 4 h,
80% and 50%.
10
Abstract
in CH2Cl2, where 13 was diacetylated product and 14 was triacetylated product obtained
under same reaction conditions.
The deprotection of PMB group in 13 was effected by DDQ to furnish 15
(Scheme 2), which on oxidation with IBX furnished lactol 16 along with α,β-unsaturated
aldehyde 17. Lactol 16 was acetylated with Ac2O, Et3N and DMAP in CH2Cl2 to furnish
1 and 2.
In a further study, deprotection of PMB group in 8 was effected with DDQ to
furnish 18 (Scheme 3), which on partial hydrogenation with Lindlars12 catalyst furnished
cis-olefin 19. Oxidation of 19 with IBX furnished lactol 20, which on acetylation with
Ac2O, Et3N and DMAP in CH2Cl2 furnished 21 and 22. The cis-dihydroxylation of 21
and 22 with OsO4 and NMO afforded 23 and 24, which on acetylation with Ac2O, Et3N
and DMAP in CH2Cl2 furnished 3 and 4.
11
Abstract
Scheme 3
TBDMSO
8
TBDMSO
O
O
a
HO
HO
O
O
TBDMSO
HO
e
HO
O
TBDMSO
O
O
TBDMSO
AcO
AcO
d
O
TBDMSO
HO
O
e
O
O
O
OAc
4
O
TBDMSO
AcO
O
d
HO
O
OAc
22
20
O
OAc
23
O
O
O
OH
O
O
OAc
21
d
O
O
c
19
O
O
O
OH
18
TBDMSO
O
b
O
OH
TBDMSO
O
OAc
24
O
O
AcO
O
O
OAc
O
3
Reagents and conditions: a) DDQ, CH2Cl2:H2O (19:1), rt, 6 h, 72%; b) H2, Pd/CaCO3, quinoline,
n-hexane, rt, 6 h, 80%; c) IBX, DMSO, 0 oC, 6 h, 70%; d) Ac2O, Et3N, DMAP, CH2Cl2, 0 oC, 6 h,
90%; e) OsO4, NMO, acetone: water (3:1), rt, 15 days, 80%, 85%.
Synthesis of C-linked 4-deoxy spiro disaccharides through ring closing
metathesis
Having achieved successful synthesis of C-linked 2-deoxy spiro disaccharides, the
study was then extended to stereoselective synthesis of C-linked 4-deoxy spiro
disaccharides 25 and 26 (Figure 2).
The main strategy in the present study involves the preparation of bis-allylic
derivatives from ulose, their conversion into enones via RCM protocol and
functionalisation of ‘active olefinic site’ in pyran to develop the new saccharide moiety.
12
Abstract
Accordingly, known ulose derivative 7 (Scheme 4) on Luche reaction conditions
with allyl zinc bromide gave carbinol 27. Alkylation of 27 with allyl bromide and NaH
furnished 28, which was exposed to Grubbs’ catalyst [bis(tricyclohexylphosphine)benzylidene ruthenium(IV)dichloride]13 leading to the exclusive formation of the pyran
29 via RCM. Allylic carbon adjacent to the oxygen in 29 was oxidized with PDC and
NaOAc to afford lactone 30, which on finally, cis-dihydroxylation with OsO4 and NMO
afforded the 4-deoxy spiro disaccharides 25 and 26.
Chapter III: Some Useful Synthetic Transformations
Section A: A Versatile and Practical Synthesis of bis(indolyl)methanes/
bis(indolyl)glycoconjugates Catalysed by Trichloro-1,3,5-triazine
This section deals with the synthesis of bis(indolyl)methanes/bis(indolyl)glycoconjugates
catalysed by trichloro-1,3,5-triazine as a Lewis acid catalyst.
Due to the potent biological activity exhibited by various indole derivatives, there
is a continuous demand for novel synthetic methods in this area. Bis(indolyl) methanes
are gaining prominence in view of their occurrence in bioactive metabolites of terrestrial
and marine origin. The azafulvenium salts obtained from electrophilic substitution of
indoles with aliphatic or aromatic aldehydes or ketones undergo further substitution to
13
Abstract
afford bis(indolyl)methanes. Bis(indolyl)methanes have been obtained by reactions of
indoles with various aldehydes or ketones in the presence of either protic or Lewis acids.
Most of the previously reported methods suffer from several setbacks such as
requirement of a stoichiometric amount of the Lewis acid, expensive and highly toxic
catalysts, long reaction times. However these problems were overcome to some extent by
recently reported methods, the use of an ionic liquid and the use of I2. Hence a more
efficient and practical alternative using an inexpensive and environmentally friendly
reagent is still warranted. Herein, we introduced a new catalyst for the synthesis of
various bis(indolyl)methanes and bis(indolyl)glycoconjugates by reaction of indoles with
a variety of aldehydes in acetonitrile using trichloro-1,3,5-triazine (TCT) (10 mol%) as a
catalyst at room temperature (Scheme 1).14
Scheme 1
R
TCT (10 m ol%)
N
H
+
R-CHO
CH3CN, rt
N
H
N
H
R = alkyl, aryl, sugar
Benzaldehyde (entry 1, Table 1) on reaction with indole with 10 mol% of TCT in
acetonitrile furnished 1a in good yield. Similarly p-nitrobenzaldehyde (entry 4)
possessing an electron-withdrawing group underwent smooth reaction under the above
reaction conditions to afford 4a. These reactions prompted us to extend the scope of TCT
as a catalyst for the synthesis of various bis(indolyl)methanes. Aromatic (entries 2, 3),
aliphatic (entry 7), alicylic (entry 8), heteroaromatic (entries 5, 6,) and ,-unsaturated
(entry 9) aldehydes similarly gave the corresponding bis(indolyl)methanes 2a, 3a, 5a-9a
within 10-15 min. It is noteworthy that all the substrates reacted with equal ease in short
times, furnishing the products in high yields and with no side products. Further, the
reaction of a ketone (entry 10) gave the bisindole 10a, albeit requiring 10 h.
In continuation of our work on C-nucleosides and new glycosubstances we turned
our attention to the construction of bis(indolyl)glycoconjugates from sugar aldehydes.
Accordingly, sugar derived aldehyde, 1,2-O-isopropylidene-3-O-methyl--D-xylo-pentodialdo-1,4-furanose (entry 11) underwent a facile reaction with indole to give the Clinked glycoconjugate 11a. 2,5-Anhydro-3,4:6,7-di-O-isopropylidene aldehydo-Dglycero-D-galacto-heptofuranose (entry 12) and 2,5-anhydro-6-O-t-butyldimethylsilyl-
14
Abstract
3,4-O-isopropylidene-D-allose (entry 13) also successfully reacted with indole to furnish
bis(indolyl) C-nucleoside glycoconjugates 12a and 13a respectively (Table 1).
Under the above reaction conditions, indole-3-acetic acid on reaction with pmethoxybenzaldehyde gave 14a, the electrophilic substitution taking place at C-2 as C-3
was blocked (Scheme 2).
Scheme 2
HOOC
N
H
14
COOH
HOOC
CHO
TCT, CH3CN, rt
+
2h
N
H
N
H
OCH3
3
14a (87%)
OCH3
The plausible mechanism of the reaction is as shown below (Figure 1). The
‘insipient’ moisture reacts with TCT to release 3 moles of HCl and cyanuric acid
(removable by water washing) as byproduct. The in situ generated HCl acts as a protic
acid to activate the carbonyl oxygen to form the bis-indole derivatives.
Figure 1
H + H
O
ClN
N
Cl
N
:OH2
N
OH
N
_
Cl
N
Cl
15
Cl
N
HCl
Cl
16
Cl
OH
N
N
17
N
Cl
HO
N
N
+ 3 HCl
OH
18
To determine the role of ‘insipient’ moisture for ‘in situ’ HCl generation from
TCT, reaction was carried out on 3 in the presence of MS 4Ao and found there was no
reaction even after 24 h. However, when all the ingredients of the reaction were
azeotropically dried and utilized, the reaction was very sluggish and less than 20%
conversion was observed even after 24 h. These two experiments thus amply indicate that
‘insipient’ moisture is essential to release ‘HCl’ from TCT.
In summary, we have developed an efficient TCT-catalyzed protocol for the
synthesis of bis(indolyl)alkanes and bis(indolyl)glycoconjugates in short reaction times
under mild reaction conditions in high yields. The readily available inexpensive TCTcatalyst makes this protocol an attractive method particularly for the synthesis of unusual
C-nucleoside conjugates.
15
Abstract
Table 1: TCT (10 mol%) Catalysed Synthesis of Bis(indolyl)methanes/Bis(indolyl)glycoconjugates
Entry
Aldehyde
Product
R CHO
R
Time
(min)
yield
(%)
15
92
10
94
In
R = phenyl
1
R = p-chloro phenyl
2
In
R = phenyl
1a
R = p-chloro phenyl
2a
3
R = p-methoxy phenyl
3
R = p-methoxy phenyl
3a
10
90
4
R = p-nitro phenyl
4
R = p-nitro phenyl
4a
10
89
10
90
10
88
15
87
10
86
10
70
10 h
85
30
85
40
72
45
72
1
2
In
5
S 5 CHO
S 5a
In
In
6
N
N
CHO
6a In
In
6
7
CHO
7a
7
In
In
8
CHO
8a In
In
8
9
CHO
Ph
9
Ph
9a
O
CH3
In
In 10a
10
10
11
OHC
O
12
In
O
O
11
O
O
O
O
In
O
MeO
In
CHO
O
MeO
O
11a
O
In
O
O
O 12
O
In
O 12a
In
13
O
TBSO
O
CHO
O
TBSO
O 13
O
In
13a
O
* In = 3-Indolyl
16
Abstract
Section B: Rapid and Facile Lewis Acid Catalysed Boc Protection of
Amines
This section deals with Zirconium(IV) chloride (10 mol%) catalyzed Boc protection of
amines, amino acids, sugar β-amino acids with (Boc)2O in acidic conditions.
Among the various protecting groups used for amines, Boc protection has become
a fundamental tool of modern peptide synthesis and particularly of the Merrifield strategy
for solid-phase peptide synthesis. Owing to the instability of the corresponding t-butyl
chloroformate and the explosive properties of t.butylazido formate, the (Boc)2O reagent
is widely used for the introduction of the t-butoxycarbonyl group. Even though a variety
of base mediated reaction conditions are available for Boc protection, the only reported
acid (Yttria-Zirconia) mediated reaction conditions need longer reaction times (3-48 h).
In pursuance of our work on new synthetic methods and non-natural peptides, we were
interested in exploring the possibility of developing Lewis acid catalysed reaction
conditions for Boc protection. Herein, we introduce the ZrCl4 catalysed Boc protection of
amines with short reaction times and high yields (Equation 1).15
Equation 1
R-NH 2
(Boc)2O, ZrCl4 (10 mol%)
R-NHBo c
CH3CN, rt
Aniline (entry 1, Table 1) and (Boc)2O in acetonitrile were treated with 10 mol%
ZrCl4 at room temperature to afford 1a in 3 min. The same reaction with Yttria-Zirconia
as catalyst took 14 h, while it required 48 h in the absence of any catalyst. This
interesting result prompted us to explore the reactivity of (Boc)2O with a variety of
amines in the presence of ZrCl4. Accordingly, aryl/heteroarylalkyl (entries 2 and 3),
cycloalkyl (entries 4 and 5), acetoxyalkyl amines (entry 8) and secondary amines (entries
9, 10 and 11) underwent smooth Boc protection to furnish the corresponding products
(Boc protected amines) in good to excellent yields (Table 1) in 3-10 min. It is worth
mentioning that aminols (entries 6 and 7) on treatment with (Boc)2O in acetonitrile at
room temperature chemo selectively gave 6a and 7a in high yields.
17
Abstract
Table 1: ZrCl4 (10 mol%) Catalysed Boc Protection of Amines with (Boc)2O
Entry
Starting material
product
Time
(min)
Yield
(%)
RNHBoc
RNH2
1
R = phenyl (1)
R = phenyl (1a)
3
95
2
R = benzyl (2)
R = benzyl (2a)
3
96
R = 4-pyridyl methyl (3a)
3
90
R = cyclohexyl (4a)
3
91
5
88
5
96
5
90
10
85
5
92
3
4
R = 4-pyridyl methyl (3)
R = cyclohexyl (4)
5
NH 2
N
NHBo c
N
5a
5
6
NH 2
HO
NHBo c
6a
HO
6
NH 2
7 R' = H
OR'
NHBo c
7a R' = H
8 R' = Ac
8a R' = Ac
OR'
7
8
9
N
X
N
NH
9
NBoc
9a
X
NH
NBoc
10
11
10 X = CH2
10a X = CH2
5
92
11 X = O
11a X = O
10
90
In a further study, amino acid esters (entries 1, 2 and 3, Table 2) were converted
to the corresponding N-Boc esters under similar reaction conditions in 10 min and in
good yields. However, the methyl ester of histidine (entry 5, Table 2) on reaction with
one mole of (Boc)2O gave 16a as the sole product in 10 min, while with 2 moles it gave
16b in 15 min. The chemoselective protection of cysteine (entry 4, Table 2) gave the NBoc protected derivative 15a in 10 min. This procedure could conveniently be applied to
a variety of amino acids. In a continued study, the C-linked carbo β-amino acid esters
derived from sugars (entries 6, 7 and 8, Table 2) underwent smooth Boc protection in
18
Abstract
Table 2: ZrCl4 (10 mol%) Catalysed Boc Protection of Amino Acid Esters with (Boc)2O
1
Yield
(%)
10
82
10
84
10
80
10
81
16a R = H
10
16b R = Boc
15
82
80
25
88
15
93
20
89
product
NH 2
MeO2C
MeO2C
12
13
MeO2C
CO2Me
13a
NH 2.HCl
NHBo c
14
HS
15
12a
CO2Me
CO2Me
MeO2C
CO2Me
14a
NHBo c
NH 2.HCl
4
NHBo c
NHBo c
NH 2
2
3
Time
(min)
Starting material
Entry
HS
CO2Me
15a
CO2Me
CO2Me
CO2Me
5
HN
N
NH 2.HCl
16
O
6
O
NH 2
O
MeO
N
RN
O
MeO
7
NH 2
O
O
MeO
8
OMe
MeO
O
O
O
MeO
O
O
O
NHBo c
O
O
19
O
O
O
O 19
OMe
O 18a
O
NH 2
MeO
NHBo c
O
O 18
O
O
NHBo c
O
MeO 17a O
MeO 17 O
O
NHBo c
19a
Abstract
the presence of ZrCl4 (10 mol%) in 15-25 min to give 17a, 18a and 19a respectively, in
high yields. The conventional procedure [(Boc)2O, Et3N, THF] for the same
transformation took more than 2 h.
In conclusion, this protocol is operationally simple, rapid and high yielding. The
reaction conditions are mild and inexpensive involving the use of a readily available and
environmental friendly catalyst (ZrCl4) at room temperature. The formation of side
products was not observed.
Section C: An Efficient Solvent Free Protocol for the Synthesis of 4Substituted Coumarins Using Zirconium(IV) Chloride
This section deals with the synthesis of 4-substituted coumarins via Pechman reaction
using 10 mol% ZrCl4 in solvent free conditions treating phenols with β-ketoesters.
Coumarins are featured widely in a variety of pharmacologically and biologically
active compounds. In addition, they also constitute a structural unit of a series of natural
products. They are used as anticoagulants, additives in food and cosmetics and in the
preparation of insecticides, optical brighteners, laser dyes, dispersed fluorescent
compounds and frequently encountered as signaling units in sensors and in sophisticated
photophysical systems. Thus, synthesis of this heterocyclic nucleus is of much current
importance. Several methods have been developed to synthesize coumarins, while many
of these methods utilized acids such as H2SO4, trifluroacetic acid, phosphorous
pentoxide, Lewis acids, ionic liquids, solid catalysts and microwave irradiation. However,
most of these procedures suffer from the disadvantages such as elevated temperatures,
longer reaction times, poor yields and undesirable side products. In connection with of
our work on calanolides,16 a novel coumarin based non-nucleoside HIV-RT inhibitor and
aza-calanolides,17 we were interested to explore the scope of ZrCl4 as an efficient acid
catalyst.18 Herein, we developed a rapid, efficient and convenient solvent free route for
the synthesis of coumarins using ZrCl4 (Equation 1).
Equation 1
R'
O
R
O
ZrCl4 (10 mol%)
+ R'
OEt
OH
R' = CH3, CH2Cl, Ph, Fu
20
rt
R
O
O
Abstract
In the first case, an equimolar quantity of resorcinol 1 (entry 1) and ethyl
acetoacetate were treated with catalytic amount of ZrCl4 (10 mol%) at room temperature
for 5 min. to give the corresponding coumarin 1a (m. p. 184-185 oC, {lit. m. p. 185 oC}).
To characterize 1a, it was treated with AcCl in pyridine to form the corresponding acetate
1a’. In 1HNMR of 1a’ the acetyl signals resonated at 2.25 as a singlet, while the –CH3
and olefinic protons resonated at 2.40 and 6.28 respectively as singlets.
Later resorcinol 1 was treated with a variety of -ketoesters viz., ethyl 4chloroacetoacetate, ethyl benzoacetate and ethyl furoacetate (entries 2, 3 and 4) to furnish
the corresponding coumarins 1b-1d in good yield within 5-10 min. Compounds 1b, 1c
and 1d indicated the m. p. 180-181 oC, 256-257 oC and 210-212 oC corresponding the
reported m. p. 181 oC, 256.5-257.5 oC and 212 oC respectively in literature. Encouraged
by this result, other substrates were subjected to similar reaction conditions in an effort to
elucidate the scope of ZrCl4 as a new catalyst for coumarin synthesis. Accordingly,
pyrogallol 2, phloroglucinol 3, 2-methyl resorcinol 4 and -naphthol 5 were subjected to
reaction with different -ketoesters as shown in Table 1 and Table 2, to the respective
coumarins. The yields in general were very high and the reaction times were shorter (510 min) regardless of the structural variations in phenols and -ketoesters. Most of these
coumarins were characterized from their melting points and molecular ion peaks in
EIMS.
In conclusion, the present protocol using ZrCl4 (10 mol%) describes a simple yet
rapid and efficient means of synthesizing coumarins under mild solvent free conditions in
high yields at ambient temperature. Thus, it would have immense effect for the synthesis
of a wide variation of coumarins for varied medical applications.
21
Abstract
Table 1: ZrCl4 (10 mol%) Catalysed Synthesis of 4-Substituted Coumarins
1
-Keto ester
Phenol
Entry
HO
HO
OH
Time
(min)
Product
O
O
CH3COCH2CO2Et
1
2
ClCH2COCH2CO2Et
HO
O
1b
HO
1
O
COCH 2CO2Et
1
O
HO
5
95
180-181
(181)
10
91
256-257
(256.5257.5)
10
90
210-212
(212)
5
96
241-243
(243)
5
98
133-135
(133-137)
10
94
195-197
(195-197)
10
90
190
(190)
O
Ph
O
O
O
1d
OH
HO
92
CH2Cl
PhCOCH2CO2Et
1c
4
184-185
(185)
5
1a CH3
1
3
o
yield m. p. C
(%) (lit. m. p.)
O
OH
OH
HO
O
O
5
CH3COCH2CO2Et
2a
2
CH3
OH
HO
6
2
O
O
ClCH2COCH2CO2Et
2b
CH2Cl
OH
HO
7
2
2
O
PhCOCH2CO2Et
HO
8
O
2c
Ph
OH
O
O
COCH 2CO2Et
O
2d
22
O
Abstract
Table 2: ZrCl4 (10 mol%) Catalysed Synthesis of 4-Substituted Coumarins
Entry
9
-Keto ester
Phenol
HO
HO
OH
Time yield
(min) (%)
Product
O
O
CH3COCH2CO2Et
3a
3 OH
10
3
ClCH2COCH2CO2Et
OH
O
OH
HO
O
O
3c OH Ph
CH3
HO
O
OH
12
97
187-189
(189)
10
94
243-246
(246-247)
5
95
263-265
(265-266)
5
96
284-286
(285.5286.5)
10
90
284-285
(285)
10
92
153-155
(155)
10
95
165-167
(167)
O
CH3COCH2CO2Et
4
5
280-281
(280)
O
PhCOCH2CO2Et
CH3
93
CH2Cl
HO
3
11
5
CH3
HO
3b
m. p. oC
(lit. m. p.)
4a
CH3
CH3
HO
13
O
O
ClCH2COCH2CO2Et
4
4b
CH2Cl
CH3
HO
14
O
O
PhCOCH2CO2Et
4
4c
O
Ph
OH
O
CH3COCH2CO2Et
15
CH3
5a
5
O
16
5
O
ClCH2COCH2CO2Et
CH2Cl
5b
23
Abstract
REFERENCES
1.
a) Hirota, A.; Isogai, A.; Sakai, H. Agric. Biol. Chem. 1981, 45, 799; b) Hirota,
A.; Sakai, H.; Isogai, A. Agric. Biol. Chem. 1985, 49, 731.
2.
Fujii, Y.; Fukuda, A.; Hamasaki, T.; Ichimoto, I.; Nakajima, J. Phytochemistry
1995, 40, 1443.
3.
a) Smith, C. J.; Abbanat, D.; Bernan, V. S.; Maiese, W. M.; Greenstein, M.;
Jompa, J.; Tahir, A.; Ireland, C. M. J. Nat. Prod. 2000, 63, 142; b) Franck, X.;
Araujo, M. E. V.; Jullian, J. C.; Hocquemiller, R.; Figadere, B. Tetrahedron Lett.
2001, 42, 2801.
4.
Hansske, F.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. Bull. Chem. Soc
Jpn. 1978, 51, 2343.
5.
Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. Bull. Chem. Soc
Jpn. 1979, 52, 1989.
6.
Jacobsen, E. N.; Wu, M. H. In Comprehensive Asymmetric Catalysis; Jacobsen,
E. N.; Pfaltz, A.; Yamamoto, H.; Eds.; Pringer: New York, 1999; Chapter 18.2.
7.
Omura, K.; Swern, D. Tetrahedron 1978, 34, 1651.
8.
Austin, K. A. B.; Banwell, M. G.; Loong, D. T. J.; Rae, A. D.; Willis, A. C. Org.
Biomol. Chem. 2005, 3 (Article in press).
9.
a) Sharma, G. V. M.; Hymavathi, L.; Radhakrishna, P. Tetrahedron Lett. 1997,
38, 6929; b) Sharma, G. V. M.; Chander, A. S.; Krishnudu, K.; Radhakrishna, P.
Tetrahedron Lett. 1997, 38, 9051; c) Sharma, G. V. M.; Chander, A. S.;
Krishnudu, K.; Radhakrishna, P. Tetrahedron Lett. 1998, 39, 6957; d) Sharma,
G.V. M.; Chander, A. S.; Reddy, V. G.; Krishnudu, K., Rao, M. H. V. R.;
Kunwar, A. C. Tetrahedron Lett. 2000, 41, 1997; e) Sharma, G. V. M.; Chander,
A. S.; Krishnudu, K.; Radhakrishna, P.; Rao, M. H. V. R.; Kunwar, A. C.
Tetrahedron: Asymmetry 2000, 11, 2643.
10.
Sharma, G. V. M.; Reddy, J. J.; Rao, M. H. V. R.; Gallois, N. Tetrahedron:
Asymmetry 2002, 13, 1599.
11.
a) Cha, J. K; Christ, W. J.; Kishi, Y. Tetrahedron Lett. 1983, 24, 3943; b) Cha, J.
K.; Christ, W. J.; Kishi, Y. Tetrahedron 1984, 40, 2247.
12.
Lindlar, A. Helv. Chem. Acta. 1952, 35, 446
24
Abstract
13.
a) Grubbs, R. H.; Pine, S. H. in Comprehensive Organic Synthesis, Trost, B. M.;
Fleming, I.; Paquette, L. A. Eds.; Pergamon: New York 1991, Vol. 5, Chapter
9.3; b) Schrock, R. R. in The Strem Chemiker, Vol. XIV, Strem Chemicals,
Newburgport 1992, No.1, 1.
14.
Sharma, G. V. M.; Reddy, J. J.; Lakshmi, P. S.; Radhakrishna, P. Tetrahedron
Lett. 2004, 45, 7729.
15.
Sharma, G. V. M.; Reddy, J. J.; Lakshmi, P. S.; Radhakrishna, P. Tetrahedron
Lett. 2004, 45, 6963.
16.
a) Ph. D. thesis of S. Mahender Rao submitted to Osmania University on
February 1995; b) Kashman, Y.; Gustafson, K. R.; Fuller, R. W.; Cardellina, J.
H.; Mcmahon, J. B.; Currens, M. J.; Buckheit, R. W.; Hughes, S. H.; Cragg, G.
M.; Boyd, M. R. J. Med. Chem. 1992, 35, 2735.
17.
Sharma, G. V. M.; Illangovan, A.; Narayanan, V. L.; Gurjar, M. K. Tetrahedron
2003, 59, 95; US Patent No. 6, 191,279 (20 Feb., 2001).
18.
a) Sharma, G. V. M.; Reddy, Ch. G.; Radhakrishna, P. J. Org. Chem. 2003, 68,
4574; b) Sharma, G. V. M.; Reddy, Ch. G.; Radhakrishna, P. Synlett 2003, 1728;
c) Sharma, G. V. M.; Srinivas, B.; Radhakrishna, P. Tetrahedron Lett. 2003, 44,
4689.
25