Microsoft Word
advertisement
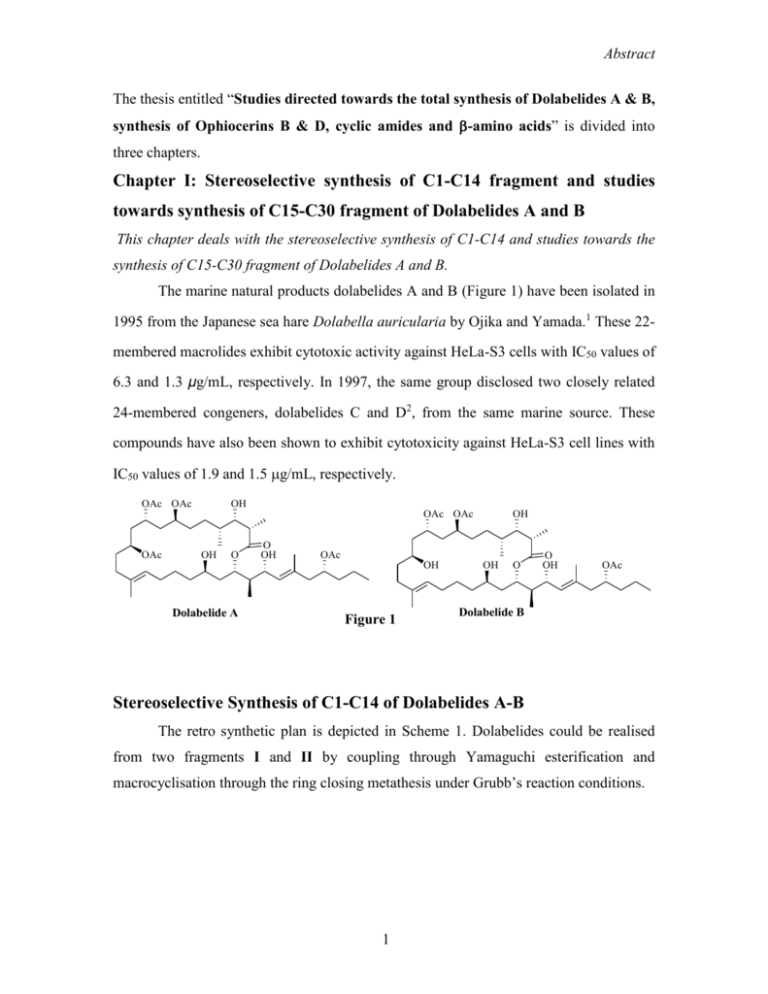
Abstract The thesis entitled “Studies directed towards the total synthesis of Dolabelides A & B, synthesis of Ophiocerins B & D, cyclic amides and -amino acids” is divided into three chapters. Chapter I: Stereoselective synthesis of C1-C14 fragment and studies towards synthesis of C15-C30 fragment of Dolabelides A and B This chapter deals with the stereoselective synthesis of C1-C14 and studies towards the synthesis of C15-C30 fragment of Dolabelides A and B. The marine natural products dolabelides A and B (Figure 1) have been isolated in 1995 from the Japanese sea hare Dolabella auricularia by Ojika and Yamada.1 These 22membered macrolides exhibit cytotoxic activity against HeLa-S3 cells with IC50 values of 6.3 and 1.3 μg/mL, respectively. In 1997, the same group disclosed two closely related 24-membered congeners, dolabelides C and D2, from the same marine source. These compounds have also been shown to exhibit cytotoxicity against HeLa-S3 cell lines with IC50 values of 1.9 and 1.5 g/mL, respectively. OAc OAc OH OAc OAc OAc OH O O OH OAc OH Dolabelide A OH Figure 1 OH O O OH OAc Dolabelide B Stereoselective Synthesis of C1-C14 of Dolabelides A-B The retro synthetic plan is depicted in Scheme 1. Dolabelides could be realised from two fragments I and II by coupling through Yamaguchi esterification and macrocyclisation through the ring closing metathesis under Grubb’s reaction conditions. 1 Abstract Scheme 1 OAc OAc OH 4 9 OAc OAc 7 1 11 OR 14 OH 2 OH O O OH 19 21 23 OR OAc 25 27 OH O OH O OAc 30 Dolabelides A: R = OAc Dolabelides B: R = H OAc OAc 9 OH 4 7 2 + 3 15 1 11 OR OH OH OH 19 21 23 OH O C1-C14 segment (I) OAc 25 27 30 C15-C30 segment (II) 14 Retro synthetic analysis of C1-C14 segment of Dolabelide A-B The retro synthetic plan is depicted in Scheme 2. The synthesis of target fragment 1 is planned by a C-C bond formation between C6 and C7 carbons via acetylene 3 on addition to the aldehyde 2, while the sub segment C1-C6 (3) could be synthesized from 5. The other sub fragment 2 could be prepared from commercially available 1,4-butane diol. Scheme 2 PMP O I OH O BnO OP 11 14 9 7 1 1 OBn 3 PMP OP O O CHO BnO + OBn 2 BnO 3a P = MPM 3b P = TBS OH 4 HO OBn 5 2 Abstract Accordingly, the known epoxide 63, was derived from mono benzyl ether of 1,4butane diol 4 (Scheme 3). Epoxide 6 on treatment with Red-Al in dry THF gave diol 7, which, on reaction with anisaldehyde dimethylacetal in dry CH2Cl2 gave 8. Acetal 8 on treatment with DIBAL-H, followed by Swern4 oxidation of 9 gave the aldehyde 10, which on further reaction with (methoxycarbonylmethylene)triphenyl phosphorane in benzene at reflux afforded ester 11. Reduction of ester 11 with DIBAL-H gave allyl alcohol 12. Stereoselective epoxidation of allyl alcohol 12 with (+)-DIPT furnished epoxide 13 (de 97:3). Further, epoxide 13 on treatment with Red-Al furnished diol 14, which on oxidative acetalization with DDQ,5 gave acetal 15. Finally, oxidation of terminal alcohol in 14 with Dess-Martin periodinane6 furnished aldehyde 2. Scheme 3 PMP H OH O BnO 6 a OH 7 H BnO R d OH b e BnO 9 R = CH2OH 10 R = CHO 8 PMBO R f O BnO OPMB OPMB c O BnO g H BnO O OH 11 R = CO2Me 12 R = CH2OH 13 H PMP PMBO a O OH h BnO O i BnO 2 15 14 Reagents and conditions: a) Red-Al, THF, 0 oC, 5 h, 84%; b) PMBacetal, PPTS, CH2Cl2, 0 oC-rt, 2 h, 83% ; c) DIBAL-H, CH2Cl2, CH2Cl2, 0 oC, 1 h, 86%; d) (COCl)2, DMSO, Et3N, CH2Cl2, -78 oC, 3 h, 85%; e) Ph3P=CHCO2Me, benzene, reflux, 5 h, 78%; f) DIBAL-H, CH2Cl2, 0 oC, 4 h, 83%; g) (+) DIPT, Ti(iOPr)4, cumene hydroperoxide, CH2Cl2, -20 oC, 6 h, 75%; h) DDQ, CH2Cl2, MS-4o, 0 oC-rt, 8h, 74%; i) Dess-Martin periodinane, NaHCO3, CH2Cl2, 0 oC- rt, 2 h, 86%. OH OH Oxazolidinone 16 (Scheme 4) on treatment with chloromethyl benzyl ether7 (BOMCl) in the presence of TiCl4 and Et3N in dry CH2Cl2 and subsequent reduction with NaBH4 in MeOH gave alcohol 178. Oxidation of alcohol 17 under Swern reaction conditions gave 18. For the synthesis of fragment 3a (Scheme 4), the addition of (Z)enolate, generated from 16, under Evans’ syn aldol9 protocol, by use of Bu2BOTf and iPr2EtN, to the aldehyde 18 gave 19 with excellent diastereoselectivity (de 95:5). Reduction of 19 with LiBH4 in ether resulted in diol 20, whose newly generated stereocentres were confirmed from the nOe studies of the acetonide 21. For further 3 Abstract synthesis, 20 was treated with anisaldehyde dimethyl acetal and 10 mol% of PPTS to afford 22, which on reaction with DIBAL-H gave 23. Further, oxidation of alcohol 23 under Swern oxidation conditions gave aldehyde 24, which on reaction with OhiraBestmenn diazophosphonate10 reagent resulted in acetylenic compound 3a. Scheme 4 O O O O N a, b BnO 16 Bn d OR O OBn N O R = 17 CH2OH R = 18 CHO c OH Bn e 19 OH f OBn HO O O OBn 20 21 g PMP O OPMB O OPMB h OBn HO OBn 22 i j OHC 23 3a OBn 24 o Reagents and conditions: a) TiCl4, Et3N, BnOCH2Cl, CH2Cl2, 0 C, 8 h, 83%; b) NaBH4, MeOH, 0 oC, 5 h, 82%; c) (COCl)2, DMSO, Et3N, CH2Cl2, -78 oC, 3 h, 75%; d) 16, Bu2BOTf, DIPEA, CH2Cl2, -78 oC, 3 h, 80%; e) LiBH4, ether, 0 oC, 3 h, 85%; f) 2,2-DMP, PPTS, CH2Cl2, 1 h, rt, 91%; g) PMBacetal, PPTS, CH2Cl2, 0 oC-rt, 1 h, 83%; h) DIBAL-H, CH2Cl2, 0 oC, 2 h, 87%; i) (COCl)2, DMSO, Et3N, CH2Cl2, -78 oC, 3 h, 88%; j) dimethyl-1-diazo-2oxopropylphosphonate, K2CO3, MeOH, 7 h, 75%. In a different study, the aldol product 19 was treated with TBSOTf and 2,6lutidine11 to give 25 (Scheme 5), which on reduction with DIBAL-H furnished aldehyde 26. Finally, aldehyde 26 on reaction with Ohira-Bestmann diazophosphonate9 reagent was converted in to 3b. Scheme 5 O 19 a O O OTBS OTBS N Bn b OBn 25 c OHC OBn 3b 26 Reagents and conditions: a) TBSOTf, 2,6-lutidine, CH2Cl2, 0 oC, 2 h, 92%; b) DIBAL-H, CH2Cl2, -78 oC, 1 h, 75%; c) dimethyl1-diazo-2-oxopropyl phosphonate, K2CO3, MeOH, 7 h, 74% (over two steps). 4 Abstract Attempted coupling of aldehyde 2 with 3a and 3b independently under a variety reaction conditions met with failure to afford the expected carbinol (Scheme 6) Scheme 6 PMP PMP O O CHO BnO 2 O a + OH O BnO X OP OP OBn 1 OBn 3a P = PMB 3b P = TBS Reagents and conditions: a) n-BuLi, THF, -78 oC. Cross-Metathesis approach: Having met with failure by the ynone approach to achieve the synthesis of C1C14 fragment, a different synthetic strategy was planned. According to the retro synthetic analysis (Scheme 7), the synthesis of C1-C14 fragment I could be achieved from 27, which inturn could be realised by regioselective cross metathesis (CM)12 between Scheme 7 Ph O A O OH OR 9 7 3 1 BnO 14 11 OBn 27 Ph O O OR OH BnO OBn + 28 BnO 4 29a R = TBS 29b R = H HO OH OBn 5 5 Abstract fragments 28 and 29. The syn 1,3-diol in 28 could be attained by oxa-Michael13 addition reaction, which inturn could be prepared from commercially available 1,4-butane diol. Similarly, other sub segment 29 could be achieved from oxazolidinone 16 by aldol reaction under Crimmins protocol.14 Accordingly, ester 11 (Scheme 8), on oxidative deprotection of PMB ether with DDQ in aq. CH2Cl2 furnished alcohol 30, which on conjugate addition of a hemiacetal anion made in situ from benzaldehyde in the presence of a catalytic amount of potassium tertbutoxide,13 led to ester 31. Reduction of 31 with DIBAL-H at -78 oC gave aldehyde 32 which on treatment with vinylmagnesium bromide furnished 33 as a mixture of isomers (1:1). The mixture of alcohols 33 was subjected to oxidation with Dess-Martin periodinane in dry CH2Cl2 to give 34, which on reduction with Coreys’ chiral oxazaborolidine15 using [R-(CBS)] gave 28 (94% de). Scheme 8 Ph OR O O b BnO OMe a 11 R = PMB 30 R = H R c Ph O OH O e BnO d 31 R = CO2Me 32 R = CHO Ph O O BnO O O BnO 33 f 28 34 Reagents and conditions: a) DDQ, CH2Cl2:H2O, rt, 5 h, 84%; b) PhCHO, t-BuOK, THF, 0 oC, 8 h, 77%; c) DIBAL-H, CH2Cl2, -78 oC, 1 h, 68%; d) vinyl magnesium bromide, THF, 0 oC, 2 h, 84% (over two steps); e) Dess-Martin periodinane, NaHCO3, CH2Cl2, 0 oC, 2 h, 78%; f) (R)-CBS, catecholborane, toluene, -78 oC, 12 h, 76%. Aldol product 19 (Scheme 9) on reductive removal of the chiral auxiliary with DIBAL-H in CH2Cl2 at -78 oC furnished the aldehyde 35, while, the similar reaction on 25 gave 26. The aldehydes 26 and 35 were homologated with (methylene)triphenyl phosphorane in THF at 0 °C for 8 h to afford 29a and 29b respectively 6 Abstract Scheme 9 OTBS OTBS a OHC 25 OBn OBn 26 29a OH OH b OHC 19 OBn a OBn 35 29b Reagents and conditions: a) PPh3CH3I, n-BuLi, THF, 0 oC-rt, 9 h, 85%; b) DIBAL-H, -78 oC, 1 h, 90% In order to synthesize 27, the two fragments 28 and 29a (Scheme 10) were subjected to cross-metathesis reaction by using Grubbs’ 2nd generation catalyst,16 which met with failure. However, cross metathesis reaction of 28 and 29b gave the expected product 36 along with the formation of alkene dimer of 29b. Catalytic hydrogenation of 36 by use of PtO2 in EtOAc at room temperature gave 27. Scheme 10 Ph O O OH Ph BnO 28 + OTBS a O O X OH OH BnO OBn OBn 29a Cl O O NMes MesN Ph OH Cl Ph BnO 28 + OH a O O OH H 36 OBn OBn H b 29b 27 Reagents and conditions:a) Grubbs-II, CH2Cl2, 40 oC, 6 h, 65%; b) H2, PtO2, EtOAc, rt, 2 h, 85% 7 PCy3 Ph Gr-II OH BnO Ru Abstract After the successful completion of the synthesis of 27, the studies were next aimed at the synthesis of C15-C30 segment. According to the retro synthetic plan (Scheme 11), fragment 37 could be made by coupling of two sub fragments 38 and 39, while, 38 could be made from commercially available 1, 5-pentane diol derivative 40. Similarly, the synthesis of fragment 39 could be achieved starting from 3-butyne 1-ol 41a through vinyl iodide 41. Scheme 11 PMBO 15 II BnO 19 OTBS OH OMOM 21 27 23 nPr 37 PMBO OTBS OMOM CHO BnO I + nPr 38 BnO 39 OH I OH 40 41 OH 41a The known epoxide 4218 on treatment with Red-Al in dry THF gave 43 (Scheme 12). Diol 43 was treated with anisaldehyde dimethyl acetal in the presence of PPTS in dry CH2Cl2 to furnish 44, which on reduction with DIBAL-H in CH2Cl2 gave 45. Oxidation of 45 under Swern oxidation conditions and subsequent reaction of 46 under Evans syn aldol protocol using Bu2BOTf and i-Pr2EtN in CH2Cl2 gave 47. To get the required stertochemistry at C21 position, the hydroxy group at C21 in 47 was inverted by Mitsunobu reaction using DIAD and PhCO2H in THF to furnish benzoate 48, which on hydrolysis with K2CO3 in MeOH afforded alcohol 49. Protection of alcohol in 49 with TBSOTf furnished 50, which finally on reduction of the amide with LiBH4 in ether gave alcohol 51. Oxidation of alcohol 51 with Dess-Martin periodinane gave aldehyde 38. 8 Abstract Scheme 12 OH H O 42 O b a OH BnO PMP BnO OH 43 H BnO d OH PMBO OR O h f BnO Xc 47 PMBO 46 O BnO Xc g 48 R = COPh 49 R = H PMBO OTBS O OTBS j i BnO e CHO BnO OH 45 PMBO 44 OPMB OPMB c BnO O BnO Xc OH 50 38 51 o o Reagents and conditions: a) Red-Al, THF, -20 C to 0 C, 4 h, 91%; b) PMBacetal, PPTS, CH2Cl2, 0 oC, 2 h, 80%; c) DIBAL-H, CH2Cl2, 0 oC, 2 h, 80%; d) (COCl)2, DMSO, Et3N, CH2Cl2, -78 oC, 3 h, 79%; e) 16, Bu2BOTf, i-Pr2NEt, CH2Cl2, -78 oC, 4 h, 89%; f) PhCO2H, DIAD, Ph3P, THF, 0 oC, 8 h, 64%; g) K2CO3, MeOH, rt, 4 h, 68%; h) TBSOTf, 2, 6-lutidine, CH2Cl2, 0 oC, 1 h, 94%; i) LiBH4, ether, 0 oC, 2 h, 86%; j) Dess-Martin periodinane, NaHCO3, CH2Cl2, 0 oC, 2 h, 85%. The other sub fragment 39 was prepared starting from commercially available 3butyne-1-ol 52. Accordingly, 52 (Scheme 13) on zirconocene-promoted carboalumination17 and quenching with iodine resulted the corresponding vinyl iodide 41, which on oxidation under Swern oxidation conditions gave 53. Grignard reaction of 53 with n-propyl magnesium iodide in THF gave 54, which on oxidation gave the ketone 55. Scheme 13 OH ref. 17 OH 41 52 b a I OH CHO 53 O c I I 54 55 OH d I e I 56 OMOM I 39 o Reagents and conditions: a) (COCl)2, DMSO, Et3N, -78 C, 3 h, 81%; b) n-propyl iodide, Mg, THF, 0 oC, 4 h, 75%; c) DessMartin periodinane, NaHCO3, CH2Cl2, 0 oC, 2 h, 92%; d) (R)-CBS, catecholborane, toluene, -78 oC, 12 h, 76%; e) MOMCl, i-Pr2NEt, CH2Cl2, 0 oC-rt, 8 h, 82%. Selective reduction of 55 with Coreys’ chiral oxazaborolidine15 [(R)-CBS] furnished 56 with required stereochemistry. Finally, alcohol 56 was protected on reaction with MOMCl to furnish 39. 9 Abstract Chapter II: Stereoselective Total Synthesis of Ophiocerin-B and D Section A: Total synthesis of Ophiocerin-D This section deals with the stereoselective total synthesis of Ophiocerin-D from D-xylose. The chemical investigations on fresh water aquatic fungi by Gloer et al.20 resulted in the isolation of tetrahydropyran derivatives ophiocerins A-C and ophiocerin D (1) from Ophioceras venezuelense (Magnaporthaceae).21 The structural analysis of ophiocerin AD was arrived at by Gloer et al. from the spectral studies, while, the absolute stereochemistry was assigned by CD spectrometry by the use of excitation chirality method. The retro-synthetic analysis of 57 (Scheme 15) indicated that it could be prepared from 58 and butynoic acid 59, while, 58 in turn could be prepared from D-xylose derivative 60. Thus, the synthetic strategy would be: a) deoxygente C-5 to methyl group, b) retention of C3/C4 for C5/C6 of 57 and c) introduction of C3/C4 diol of 57 by asymmetric dihydroxylation. Scheme 15 Yamaguchi protocol OH O Deoxygenation OMOM OH HO O OMOM O O O O O 57 58 O O 60 Wittig/dihydroxylation + COOH 59 Accordingly, the alcohol22 61 (Scheme 16) on protection with benzyl bromide gave 62. The benzyl ether 62 on acid (5% H2SO4, THF) catalyzed hydrolysis at room temperature gave 63. Oxidative cleavage of 63 with H5IO6 in EtOAc at room temperature afforded the aldehyde 64, which on concomitant Wittig olefination furnished the ester 65. Treatment of 65 with TBDMSCl and imidazole in CH2Cl2 gave TBS ether 66. Asymmetric dihydroxylation23 of ester with AD-mix- gave the diol 67, which on protection with MOMCl in the presence of DIPEA afforded the MOM ether 68. Having established all the four stereocentres with absolute stereochemistry, next it was aimed at the cyclization and introduction of isocrotonyl side chain at C-5 OH group. 10 Abstract Accordingly, ester 68 on reduction with LAH in THF at room temperature gave the alcohol 69, which on further reaction with p-TsCl (Et3N, CH2Cl2) furnished tosylate 70. Tosylate 70 was exposed to TBAF at room temperature to result in desilylation and Scheme 16 O O O OH OH c b O HO O O a BnO O BnO 62 63 CHO 64 OBn OH 61 OR TBSO O OR O f, g d, e TBSO OMe OMe h, i OR OBn OR 67 R = H 68 R = MOM OBn 65 R = H 66 R =TBS OMOM OBn OMOM 69 R = H 70 R = Ts Reagents and conditions: (a) BnBr, NaH, THF, 0 oC-rt, 4 h, 87%; (b) 5% H2SO4, THF, 40 oC, 8 h, 71%; (c) H5IO6, EtOAc:H2O, 0 oC, 5 h, 82%; (d) Ph3P=CHCO2Me, benzene, reflux, 4 h, 75%; (e) TBDMSCl, imidazole, CH2Cl2, rt, 5 h, 92%; (f) AD-mix-, BuOH, rt, 12 h, 62%; (g) MOMCl, DIPEA, CH2CL2, rt, 8 h, 91%; (h) LAH, THF, 0 oC-rt, 1 h, 89%; (i) p-TsCl, Et3N, CH2Cl2, 0 oC-rt, 3 h, 68%. concomitant cyclization in one-pot to give the cyclized product 71 in 78% yield. TBAF24 in the present study acted as a desilylating agent, besides as a base to promote a facile cyclization reaction (Scheme 17). Scheme 17 OMOM RO OMOM OMOM a O OMOM c b O O 71 R = Bn 60 R = H O 72 OMOM O OMOM d 57 O O 73 (j) TBAF, THF, rt, 5 h, 78%; (k) H2, Pd(OH)2, MeOH, rt, 5 h, 87%; (l) 2-butynoic acid, 2,4,6trichlorobenzoyl chloride, Et3N, THF, DMAP, toluene, rt, 10 h, 79%; (m) Lindlar catalyst, MeOH, rt, 2 h, 75 %; (n) t-BuOH, PPTS, 70 oC, 3 h, 71%. For the introduction of the side chain, the benzyl group in 71 was removed by hydrogenation using Pd(OH)2 in MeOH to give the alcohol 60. Further, acylation of 60 11 Abstract with 2-butynoic acid 59 under Yamaguchi reaction25 conditions gave 72 in 79% yield, which on selective hydrogenation in the presence of Lindlar catalyst26 afforded 73. Finally, PPTS catalyzed deprotection of MOM groups in 73 furnished ophiocerin D 57. The optical rotation value [α]D +38.4 of synthetic 57 was matching with that of natural product [α]D +40. Section B: Stereoselective total synthesis of Ophiocerin-B This section deals with the stereoselective total synthesis of Ophiocerin-B from L-malic acid. The retro-synthetic analysis of 74 (Scheme 18) indicated that ophiocerin-B could be prepared from 75, while 75 in turn could be prepared from L-malic acid. Thus, the synthetic strategy would be: a) deoxygenation of γ-hydroxy group to methyl group b) introduction of C3/C4 of 74 diol by Sharpless asymmetric dihydroxylation. Scheme 18 OH OTBS O OH OH O O HO OH OMe O ophiocerin B 75 O O L-malic acid 74 Accordingly, the known alcohol 7627 (Scheme 19) derived from L-malic acid, on oxidation under Swern reaction conditions gave aldehyde 77, which on Wittig olefination with (ethoxycarbonylmethylene)triphenyl phosphorane in benzene reflux furnished ester 78. Scheme 19 OTBS L-malic acid Ref. 27 a, b R OTBS CO2Et 76 R = CH2OH 77 R = CHO OTBS O OH TBSO c 78 CO2Et d CO2Et O 75 Reagents and conditions: a) (COCl)2, DMSO, Et3N, CH2Cl2 -78 oC, 3 h, 84%; b) Ph3P=CHCO2Et, benzene, reflux, 4 h, 75%; c) AD-mix-, t-BuOH, rt, 12 h, 65%; d) 2,2-DMP, PPTS, CH2Cl2, rt, 2 h, 85%. 79 OH Asymmetric dihydroxylation of 78 using AD-mix-β and catalytic amount of methane sulfonamide in t-BuOH/H2O (1:1) (Scheme 19) furnished diol 79. Acetonation 12 Abstract of vic-diol with 2,2-DMP and catalytic PPTS gave 75, which on reduction with DIBALH furnished 80. Tosylation of 80 with p-TsCl and Et3N in CH2Cl2 gave 81, which on reaction with TBAF in dry THF gave 82. In the present study, TBAF acted as a desilylating agent besides as a base to promote cyclisation in single step to afford 82. Finally, deprotection of acetonide using PTSA in MeOH gave 74. Scheme 20 O a 75 O O TBSO O c d OR 74 O 80 R = H 81 R = Ts b 82 Reagents and conditions: a) DIBAL-H, CH2Cl2, 0 oC, 2 h, 86%; b) p-TsCl, Et3N, CH2Cl2, rt, 4 h, 78%; c) TBAF, THF, rt, 10 h, 65%; d) PTSA, MeOH, rt,4 h, 85%. Chapter III: Synthesis of cyclic amides and -amino acids Section A: Synthesis of cyclic amides This section deals with the synthesis of cyclic amides from D-mannose The cyclic amides that stack on top of one another forming nanotube-like structures have been the subject of many investigations due to their utility in chemical, biological and materials science.28 These cyclic oligomers, composed of carbohydrate O O NH O HN NH O O O NH O 83 N O O O O O O O O O 86 O O O O O O O O HN O O O O NH HN O Figure 2 13 N 85 O O NH HN NH O 84 O O O HN NH O O O O O HN O O O HN O HN O O O O O O O NH O N N 87 O O Abstract units, have been used in the areas of drug delivery, asymmetric synthesis and also as enzyme mimetics.29 Vancomycin, a representative of the glycopeptide class of antibiotics,30 is being used for over four decades as a weapon of last resort to combat bacterial disease. The cyclic peptides adopt antiproliferative activity by inhibiting the growth of several cancer cell lines31 and cyclic peptides form tublar ion-conducting channels in phospholipid layers.32 Kessler et al.33,34 prepared cyclic peptides using constrained sugar amino acid and β-hGly alternatively, which showed symmetry in acetonitrile. In the present study cyclic amides 83-87 (Figure 2) were prepared. Accordingly, known 2, 3; 5, 6 di-O-isopropyledine D-mannose 88, (Scheme 20) obtained from commercially available D-mannose, was treated with trimethylsulfoxinium iodide in the presence of potassium tert. butoxide in dry DMSO at room temperature for 8 h to afford mannose methonal 89. Conventional tosylation of primary alcohol 89 with p-TsCl, Et3N and catalytic DMAP in CH2Cl2 at room temperature for 5 h gave 90. Tosylate 90 was further treated with NaN3 in DMF at 80 C for 8 h to afford azide 91, which on reduction with LAH in dry THF furnished 92. Scheme 20 O O O OH O O OH b a D-Mannose O O O O O 88 d OTs c O O O 90 89 O O O O O O N3 O O O e O NH2 O 91 O 92 Reagents and conditions: a) cat. H2SO4, Dry CuSO4, Acetone, rt, 12 h, 92%; b) TMSOI, t-BuOK, DMSO, rt; 3 h, 86% c) TsCl, Et3N, DMAP, CH2Cl2, rt, 3 h, 83%; d) NaN3, DMF, 80 oC, 8 h, 65%; e) LAH, THF, 0 oC, 2 h, 76%. Condensation of amine 92 (Scheme 21) with isophthaloyl chloride 97 gave 93 as a major product, while with pyridine 2,6-dicarbonyl dichloride 98, it furnished 94 as a major product. The diacetonides in 93 and 94 were hydrolysed to give the respective diols 93a and 94a which were subjected to oxidative cleavage to give aldehydes 95 and 14 Abstract 96, aldehydes 95 and 96 on further reduction with NaBH4 gave alcohols 95a and 96a, which were treated with MsCl and Et3N in dry CH2Cl2 to furnish the corresponding mesylates, which, on further reaction with NaN3 in DMF furnished respective azides 99 and 100. Finally, reduction of the azides 99 and 100 with Ph3P in MeOH furnished 101 and 102 respectively. Scheme 21 O NH 92 a O X O HN NH O O O O O O O O OH O O 93 X = CH 94 X = N X NH c, d O O O O R HO 93a X = CH 94a X = N HN O O e, f O O O R 95 X = CH; R = CHO 95a X = CH; R = CH2OH 96 X = N; R = CHO 96a X = N; R = CH2OH O X NH HN O O HO OH O O O O O O HN O b O O X g O O R1 R1 1 99 X = CH, R = N3; 100 X = N, R1 = N3 101 X = CH, R1 = NH2; 102 X = N, R1 = NH2 Reagents and conditions:a) isophtaloyl chloride/2,6-dicorbonyl dichloride, Et3N, CH2Cl2, rt, 6 h, 85%; b) PTSA, MeOH, rt, 1 h, 80%; c) NaIO5, CH2Cl2, rt, 6 h, 75%; d) NaBH4, MeOH, 0 oC-rt, 8 h, 84%; e) MsCl, Et3N, CH2Cl2, 0 oC, 4 h, 75%; f) NaN3, DMF, 80 oC, 4 h, 78%; g) , LAH, THF, rt, 1 h, 75%. For the synthesis of the cyclic amides 83 and 85 the common precursor was 101. Thus, condensation of 101 with 97 gave 83, while, with 98 it gave 85. Similarly, condensation of 102 with 98 afforded 84. Likewise, the other two cyclic amides 86 and 87 (Scheme 22) were prepared from diol 95a, wherein condensation of 95a with 97 gave 86, while with 98 it furnished 87. 15 Abstract Scheme 22 101 a 83 a 102 b b 84 86 O 95a b 85 87 X Cl O Cl 97 X = CH 98 X = N Reagents and conditions: a) 97, Et3N, CH2Cl2, 0 oC, rt, 6 h, 35%; b) 98, Et3N, CH2Cl2, 0 oC, rt, 7 h, 30% The above cyclic amides 83-87 showed metal binding with Li, Na, K, Rb and Cs. Further, Li and Na have shown strong binding with these cyclic amides. The MSMS studies showed Li and Na binding inside the cavity of the cyclic amides while, K, Rb and Cs were found not binding strongly, indicating only peripheral binding with the cyclic amides. Section B: An efficient ZrCl4 catalyzed aza-Michael addition reaction: synthesis of C-linked carbo β3-amino acids This section deals with the synthesis of C-linked carbo -amino acids using ZrCl4 as a Lewis acid catalyst The synthesis of β-amino acids,35 which are constituents of several natural products and useful starting materials for the synthesis of bioactive compounds, has received much attention in the recent past.36 β-Peptides,37 the non-natural oligomers of βamino acids, are targeted as potential peptidomimetics,38 since they are stable to peptidases and conformationaly more rigid unlike peptides derived from α-amino acids. The presence of an additional methylene group in them provides an opportunity to create skeletal as well as stereochemical diversity. A new method was envisaged for the efficient preparation of β- amino acids by aza-Michael addition. Accordingly, to evaluate the validity of ZrCl4 (10 mol%) as a catalyst for azaMichael addition using the of aromatic amines and methyl acrylate as Michael acceptor gave the dialkylated amine, while, acrylonitrile formed the mono alkylated product 16 Abstract (Scheme 23). The method has been established with a variation of aromatic and aliphatic amines. Scheme 23 CO2Me R'NH2 / ZrCl4 (10 mol%) CO2Me R' N CO2Me RT R ' = alkyl, aryl R'NH2 / ZrCl4 (10 mol%) R CN N CN R' RT R ' = alkyl, aryl Scheme 24 O O R' BnNH2 / ZrCl4 (10 mol%) R H3CO 103 H3CO RT R'' R 104 R ' = H, R" = NHBn R' = NHBn, R" = H R = Sugar TABLE 1: ZrCl4 catalysed aza-Michael addition of g-alkoxy-a, b-unsaturated esters Entry -unsaturated ester O O R O 1 O H3CO O O O OCH3 H3CO O R1 O O 2 104e:104f 90:10 1 O O O O O O 103c 104c:104d 90:10 R1 R H3CO O O OCH3 104c R = H, R1= NHBn 104d R = NHBn, R1= H (93%) O O O O O 3 R H3CO 103b H3CO 1 104a R = H, R 1= NHBn 104b R = NHBn, R = H (92%) O 2 104a:104b 87:13 O H3CO 103a O time (h) R1 H3CO O H3CO d..r b- amino ester (Yield%) O 104e R = H, R1= NHBn 104f R = NHBn, R1= H (81%) 17 Abstract Having established the ZrCl4 catalyzed solvent free conditions for aza- Michael addition, the study was then extended to γ-alkoxy α, β-unsaturated esters 103 (Scheme 24) derived from the corresponding carbohydrate aldehydes. Accordingly, reaction of ester 103a with BnNH2 (1 eq.) at room temperature in the presence of ZrCl4 (10 mol %), was indeed very facile and gave a separable diastereomeric mixture of 104a and 104b (Table 1) in 1-2 h. This is a remarkable advancement over our earlier procedure. Further, reaction of esters 104b and 104c under ZrCl4 catalyzed conditions with BnNH2 gave 104c/104d and 104e/104f respectively as a separable mixture. References: (1) Ojika, M; Nagoya, T; Yamada, K. Tetrahedron Lett. 1995, 36, 7491. (2) Suenaga, K.; Nagoya, T.; Shibata, T; Kigoshi, H; Yamada, K. J. Nat. Prod. 1997, 60, 155. (3) Babak, B; Jennifer M, S; Veera Reddy, P. J. Am. Chem. Soc. 2004, 126, 13600. (4) Mancuso, A. J.; Huang, S.-L.; Swern, D. J. Org. Chem. 1978, 43, 2480. (5) Tomoyasu, H.; Toshiaki, S.; Daisuke, Y.; Mutsumi, M.; Yoshiaki, H.; Takanori, M.; Eisuke, K.; Satoshi, O. Tetrahedron Lett. 2007, 48, 413. (6) Dess, D. B.; Martin, J. C. J. Am. Chem. Soc. 1991, 113, 7277. (7) Evans, D. A.; Urpi, F.; Somers, T. C.; Clark, J. S.; Bilodeau, M. T. J. Am. Chem. Soc. 1990, 112, 8215. (8) Prashad, M.; Har, D.; Kim, H. Y.; Repic, O. Tetrahedron Lett. 1998, 39, 7067. (9) (a) Evans, D. A.; Bartroli, J.; Shih, T. L.; J. Am. Chem. Soc. 1981, 103, 2127. (b) Evans, D. A., Rieger, D. L., Bilodeau, M. T., Urpi, F. J. Am. Chem. Soc. 1991, 113, 1047. (c) Evans, D. A., Nelson, J. V.; Taber, T. Top. Stereochem. 1982, 13, 11. (d) Gage, J.; Evans, D. A. Org. Synth. 1990, 68, 83. (10) Ohira, S. Synth. Commun. 1989, 19, 561. (11) Crimmins, M. T.; Carrollm C.A.; King, B.A. Org. Lett. 2000, 2, 597. (12) Blackwell, H. E.; O’Leary, D. J.; Chatterjee, A. K.; Washenfelder, R. A.; Bussmann, D. A.; Grubbs, R. H. J. Am. Chem. Soc. 2000, 122, 58. (13) Evans, D. A.; Gauchet-Prunet, J. A. J. Org. Chem. 1993, 58, 2446. 18 Abstract (14) Crimmins, M. T.; King, B. W.; Tabet, E. A.; Chaudhary, K. J. Org.Chem. 2001, 66, 894. (15) Corey, E. J.; Helal, C. J. Angew. Chem. Int. Ed. 1998, 37, 1987. (16) Yokokawa, F.; Asano, T.; Shioiri, T. Org. Lett. 2000, 2, 4169. (17) Negishi, M. E.; Cynthia, L. R.; David, E. V.; Horn, M. W. J. Org. Chem. 1981, 46, 4093. (18). Jia, L.; Jin, H.Y.; Changhong, K.; Richard, P. H. Tetrahedron Lett. 2006, 47, 6121. (19). Paul, R. H. Alan, W.; Joshua, D. W.; Christopher, D. T. Org. Lett. 2008, 10, 1421. (20). Reategui, R. F.; Gloer, J. B.; Campbell, J.; Shearer, C. A. J. Nat. Prod. 2005, 68, 701. (21). Shearer, C. A.; Crane, J. L.; Chen, W. Mycologia 1999, 91, 145. (22). Sharma, G. V. M.; Chandra Mouli, Ch. Tetrahedron Lett. 2002, 43, 9159. (23) Sharpless, K. B., Amberg, W.; Bennani, Y. L.; Crispino, G. A.; Hartung, J.; Jeong, K. S.; Kwong, H. L.; Morikawa, K.; Wang, Z. M,; J. Org. Chem. 1992, 57, 2768. (24). (a) Clark, J. H Chem. Rev., 1980, 80, 429-452. (b) E. J. Corey, A. Venkateswarlu, J. Am. Chem. Soc., 1972, 94, 6190. (25) Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. Bull. Chem. Soc. Jpn. 1979, 52, 1989. (26). (a) Ho, T.-L.; Liu, S. H. Synth. Commun. 1987, 17, 969. b) Ghosh, A. K.; Wang, Y.; Kim, J. T. J. Org. Chem. 2001, 66, 8973 (27) Guang, R. Z.; Wei, L. U.; Jun, C. C. Chinese Chem Lett. 2000, 11, 663. (28) Bong, D. T.; Clark, T. D.; Granja, J. R.; Ghadiri, M. R. Angew. Chem., Int. Ed. Engl. 2001, 40, 988. (29) a) Wenz, G. Angew. Chem., Int. Ed. Engl. 1994, 33, 803. b) Breslow, R.; Dong, S. D. Chem. Rev. 1998, 98, 1997. (30) a) McMormich, M. H.; Stark, W. M.; Pittenger, G. E.; McGuire, J. M. Antibiot. Annu. 1955-1956, 606. b) Harris, C. M.; Harris, T. M. J. Am. Chem. Soc. 1982, 104, 4293. (31) Gademann, K.; Seebach, D. Helv. Chim. Acta 2001, 84, 2924. (32) Clark, T. D.; Buehler, L. K.; Ghadiri, M. R. J. Am. Chem. Soc. 1998, 120, 651. 19 Abstract (33) Gruner, S. A. W.; Truffalt, V.; Voll, G.; Locardi, E.; Stockle, M.; Kessler, H. Chem. Eur. J. 2002, 8, 4366. (34) Gruner, S. A. W.; Gyorgy, K.; Richard, S.; Venetianer, A.; Kessler, H. Org. Lett. 2001, 3, 3723. (35) Liu, M.; Sibi, M. P. Tetrahedron 2002, 58, 7991. (36) Appella, D. H.; Christianson, L. A.; Karle, I. L.; Powell, D. R.; Gellman, S. H.; J. Am. Chem. Soc. 1996, 118, 13071. (37) Seebach, D.; Overhand, M.; Kuhnle, F. N. M.; Martinoni, B.; Oberer, L.; Hommel, U.; Widmer, H. Helv. Chim. Acta 1996, 79, 913. (38) (a) Seebach, D.; Beck, A. K.; Bierbaum, D. J. Chem. & Biodiversity 2004, 1, 1111. (b) Cheng, R. P.; Gellman, S. H.; DeGrado, W. F. Chem. Rev. 2001, 101, 3219. 20