[t-BuNSiMe2Flu]TiMe2 / MAO Catalyst system
advertisement
![[t-BuNSiMe2Flu]TiMe2 / MAO Catalyst system](http://s3.studylib.net/store/data/007380514_1-e67e9c14b5dd65c217acf4a4b783a868-768x994.png)
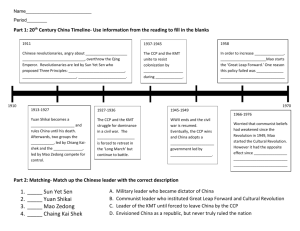
Living copolymerization of propylene and ethylene with [tBuNSiMe2Flu]TiMe2 / MAO catalyst system: Effect of MAO/Ti ratio E O Dare1,* G A Olatunji2, D S Ogunniyi2, S O Oguntoye2 and J T Bamgbose1 1Department of Chemistry, University of Agriculture, P O Box 28, UNAAB post office, Abeokuta, Nigeria 2 Department of Chemistry, University of Ilorin, P M B 1515, Ilorin, Nigeria Received 19 December 2005; revised 19 April 2006; accepted Propylene-co-ethylene polymer (Mn=528,000, Mw/Mn=1.19) was synthesized using [η1: η3-t-BuNSiMe2Flu]TiMe2 (Cat A) / methylaluminoxane (MAO) system at 0°C. Effect of MAO/Ti ratio on catalyst productivity was investigated and living nature of copolymer was maintained until the ratio reached 2000. 13C-NMR and GPC have been used to characterize the copolymer. Keywords: 13C-NMR, Copolymerization, GPC, Propylene-co-ethylene IPC Code: C08F38/02 Introduction Novel copolymers of olefins, especially block-type, having outstanding performance are in great demand in the commercial world. Copolymerization of ethylene and olefinic monomers has been studied using homogeneous catalyst systems1-5. Stereoblock ethylene/propylene copolymers are a rubbery material with the novel properties of a thermoplastic elastomer6. A melt-crystallized copolymer, where propylene was incorporated in the crystal to an extent depending on the bulkiness of the comonomer, was reported7. Homogeneous catalyst, promoting syndiotactic-specific or isotactic-specific polymerization of αolefins and a few copolymers, can be prepared by reaction of methylaluminoxane (MAO) with several transition metals (Ti, Zr and Hf) and the type of η5-ligand, which are soluble in aromatic hydrocarbon8,9. The keys to directly controlling the catalyst activity, stereospecificity and molecular weight distribution (MWD) of the polymers, lie in the titanium complex. Waymouth4 has reported the alternating copolymerization of ethylene and propylene using iPr(CpFluo)MCl2 (M = Ti, Zr). Preliminary analysis of the polymers using 13C-NMR spectroscopy revealed that the fluorenyl-based titanocenes, especially [tBuNSiMe2Flu]TiMe2, Cat A (Fig. 1) has been adopted by several authors10-13 to effect syndiospecific homopolymerization of α-olefins. t-Bu N Me Me Si Fig. 1-Cat A *Author for correspondence Tel: 234-804-313-3774 E-mail: dare3160@hotmail.com Me Ti Me A modified one-pot procedure to synthesize Cat A was reported to give a higher yield 10. This catalyst produced polypropylene in a living and syndio-specific manner. Also, Cat A has been studied10-13 for homopolymerization of α-olefin. This study reports the results of copolymerization of propylene and ethylene using Cat A in a series of experiments with systematic variation of MAO/Ti ratio. Materials and Methods Materials All operations were carried out under nitrogen atmosphere using standard Schlenk techniques. MAO was prepared following a literature procedure14. Cat A was prepared by a one-pot procedure10. Propylene and ethylene, procured from Mitsubishi Petrochemical, were purified by bubbling through a NaAlH2(i-Bu)2/1,2,3,4-tetrahydronaphthalene solution. All solvents were obtained from commercial sources and dried with standard methods. Copolymerization Propylene (1.0 mol/l) was measured by a gas flow meter and dissolved in toluene. Thereafter, prescribed amounts of cocatalyst and catalyst were added to initiate polymerization. After some time, some samples were taken out with a syringe, and then ethylene (1 atm) was introduced into the reactor. After addition of ethylene, copolymer samples were taken at regular intervals until 2.5 h when reaction was quenched by injection of acidified methanol. Copolymer obtained was washed several times with methanol and dried in vacuum at 60°C for 6h. Analytical Procedure 1 H-NMR spectra of ligands and Cat A were recorded at room temperature on a Lambda-300 spectrometer operated at 300 MHz in pulse Fourier-Transform mode. Molecular weight and molecular weight distribution of polymers were determined by Gel permeation chromatography as polystyrene (PS) standard with universal calibration, by Waters 150 CV at 140°C using O-dichlorobenzene as solvent. 13CNMR spectra were recorded at 130°C on a Lambda-500 spectrometer operated at 125.65 MHz, respectively, in pulse, Fourier-Transform mode. The spectra were obtained at 130°C in a 5.0 mm o.d. tube. In 13C-NMR measurements, pulse angle was 45°, and about 10 000 scans were accumulated in 4.9 sec of pulse repetition. Sample solution was prepared in 1,1,2,2-tetrachloroethene-d2 to contain up to 10 percent by weight. The central peak of 1,1,2,2-tetrachloroethene-d2 (74.47 ppm) was used as an internal reference. Results and Discussion Propylene Polymerization in Toluene Homopolymerization of propylene was first carried out at 0°C in toluene by batch method. Living polymerization with evidence of narrow MWD (1.08) and syndiospecificity were reestablished in line with previously reported data10. Propylene/ethylene Copolymerization Copolymerization of propylene and ethylene was conducted at 0°C with Cat A /MAO by batch method (Table 1). When Cat A was added to a solution of MAO and propylene (1.0 M) in toluene, polypropylene (mol wt, Mn=230,000) and narrow MWD (1.08) was obtained (entry 10h). Addition of ethylene to the reactor for 2.5 h produced more than two fold higher mol wt (M n=528,000) polymer with narrow MWD. Neither polypropylene nor polyethylene was produced. Copolymerization of the two monomers occurred with the same active site11. Mn value (Fig. 2) increases with increasing polymerization time while still maintaining narrow MWD. This observation further suggests that living copolymerization proceeded with catalyst system, and the catalytically active sites are uniform in the course of polymerization. However, unexpected decrease in productivity was observed during ethylene incorporation. GPC unimodal curve of the copolymer, which indicates a high block efficiency (Fig. 3) after the copolymerization, shifted to higher mol wt region. The formation of this copolymer via a uniform active Ti 4+ species by coordination mechanism was previously well established for propylene homopolymerization. Effect of the Ratio MAO/Ti on Productivity of Catalyst System Catalytic activity of Cat A in toluene9 increases with MAO/Ti ratio until a maximum ratio of 1500 (Fig. 4). Thereafter, gross decrease in activity was observed. This suggests that MAO behaves as an activator towards the initial titanocene dimethyl, and as a deactivating agent towards the titanocene active species formed. Such catalytic decrease in catalytic activity at high MAO/Ti ratio has been reported with other metallocenes9,12,13 where it was postulated that MAO might compete with the olefin for the complexation on the active sites. When MAO/Ti ratio was 2500, catalytic activity decreases drastically, and mol wt distribution became broadened to 2.10 (Table 2). MAO15,16 usually contain a significant amount of residual trialkylaluminium (TMA), which may hinder living polymerization process. Obviously, increase in MOA/Ti ratio is tantamount to an increase in residual TMA, whose excess may act as poison for the active species and thereby induce a chain transfer process through a preferential coordination of TMA with Ti species rather than counterion. This phenomenon has also been established in earlier studies13 where the effect of additives on propylene polymerization was studied. The formation of complexes between active metallocene species and TMA was observed by NMR spectroscopy16. Propylene-co-ethylene Copolymer Microstructure In 13C-NMR spectra of typical P-co-E copolymer (Fig. 5), peaks appearing at 21.78, 30.0 and 46.77 ppm correspond to propylene segments of the copolymer, whereas resonances at 27.96, 28.10 and 37.7 ppm can be assigned to methylene of ethylene segment (EEEEP) of copolymer. The triad analysis (mm=8%, mr=31% rr=61%) indicates to a large extent the syndiotacticity level of propylene segments. Furthermore, conspicuous single peaks at 20.5 (Pγγ), 29 (Tββ) and 48.5 (Sαα) signify some stereoregularity and syndiospecificity in the copolymer. Conclusions Propylene-co-ethylene copolymer synthesized from [t-BuNSiMe2Flu]TiMe2/MAO at 0°C shows that the homogeneous Cat A produces the propylene polymeric segment in a rich syndiospecific form. Cat A / MAO system gave the copolymer in a living fashion until MAO/Ti ratio exceeded 2000. Acknowledgements EOD is thankful to Prof T Shiono of TIT, Japan for providing laboratory facilities. Thanks are due to Drs Nishi and Matsumae for technical and language assistance. Appreciation also goes to UNESCO/MONBUSHO for offer of fellowship. Authors thank Prof I C Eromosele, Chemistry Department, UNAAB, Nigeria for useful suggestions. References 1 2 3 4 5 6 Resconi L, Cavallo L, Fait A & Piemontesi F, Selectivity in propene polymerization with metallocene catalysts, Chem Rev, 100 (2000) 1253-1345. Busico V, Cipullo R, Talarico G, Segre A L & Caporaso L, High-field 13C-NMR characterization of ethane/propene copolymers prepared with stereoselectivity of syndiotactic propene polymerization, Macromolecules, 31 (1998) 8720-8724. Grassi A, Ammendola P, Longo P, Albizzati E, Resconi L & Mazzocchi R, Polymerization of propene with heterogeneous metallocene/MAO, Gazz Chim Ital, 118 (1998) 539-546. Leclerc M K & Waymouth R M, Alternating ethene/propene copolymerization with a metallocene catalysts, Angew Chem Int Ed Engl, 37 (1998) 922-925. Zambelli A, Longo P, Ammendola P & Grassi A, Copolymerization of ethane/propene with some homogeneous catalysts, Gazz Chim Ital, 116 (1986) 731-737. Mirabella F M, Recent advances in metallocene-catalyzed polymerization of olefins and other monomers, Polym Mater Sc Eng, 67 (1992) 303-310. 7 8 9 10 11 12 13 14 15 16 Hosoda S, Nomura H, Gotoh Y & Kihara H, Cocrystalization of blends polymers: ethylene/propylene melt-crystallized copolymer Poymer, 31 (1990) 1999-2006. Zambelli A, Oliva L & Pellecchia C, Soluble catalysts for syndiotactic polymerization of styrene, Macromolecules, 22 (1989) 2129-2137. Ishiara N, Kuramoto M & Uoi M, Stereospecific polymerization of styrene giving the syndiotactic polymers, Macromolecules, 21 (1988) 3356-3361. Dare E O, Olatunji G A & Ogunniyi D S, Polymerization behavior of propene with [t-BuNSiMe2Flu]TiMe2: Effect of solvents and cocatalysts, Eur Polym J, 40 (2004) 2333-2341. Soga K, Chen S, Yoshiharu D, & Shiono T, Polymerization of ethylene and propylene with Cr(C 17H35COO)3/AlEt2Cl/metal chloride catalysts, Macromolecules, 19 (1986) 2893-2896. Chien J C W & Sugimoto R J, Kinetic and stereochemical control of propylene polymerization initiated by ethylene bis(4,5,5,7-tetrahydro-1-indenyl) zirconium dichloride/methyl aluminoxane catalyst, Polym Sci Part A: Polym Chem, 29 (1991) 459-470. Dare E O, Ogunniyi D S, Olatunji G A & Chattopadyay P, Polymerization of Propene with Me2Si(Me4Cp)(t-BuN)TiMe2: Effects of Trialkylaluminium as Additive Bull Chem Soc Ethiop, 18 (2004)131-136. Hassan T, Ioku A, Nishi K, Shiono T & Ikeda T, Syndiospecific living polymerization of propene with [tBuNSiMe2Flu]TiMe2 using MAO as cocatalyst Macromolecules, 34 (2001) 3142-3145. Babushkin D E, Semikolenova N V, Zakharov V A & Talsi E P, Mechanism of dimethylzirconocene activation with methylaluminoxane: NMR monitoring of intermediates at high Al/Zr ratios, Macromol Chem Phys, 201 (2000) 558-567. Bochman M & Lancaster S J, Base-free cationic zirconium benzyl complexes as highly active polymerization catalyst, Organometallics, 12 (1993) 633-640. Fig captions Fig. 1. Cat A Fig.2. Plots of Mn and Mw/Mn as a function of reaction time for propylene-ethylene copolymerization with Cat A/MAO system at 0°C by sampling method Fig. 3. GPC curves of copolymer obtained with [t-BuNSiMe2Flu]TiMe2/MAO at 0°C Fig.4. Influence of MAO/Ti ratio on the catalytic activity of Cat.A/MAO for propylene-co-ethylene polymerization in toluene at 0°C Fig. 5. 125 MHz 13C-NMR spectra of polymer obtained with [t-BuNSiMe2Flu]TiMe2/MAO at 0°C FIGURES AND TABLES t-Bu N Me Me Si Me Ti Me Mw/Mn Mn( x 10- 4) Fig. 1. Cat A Time h Fig.2. Plots of Mn and Mw/Mn as a function of reaction time for propylene-ethylene copolymerization with Cat A/MAO system at 0 oC by sampling method Activity (Kgpol/molTi.h.[P-co-E]) Fig. 3. GPC curves of copolymer obtained with [t-BuNSiMe2Flu]TiMe2/MAO at 0 oC 45 40 35 30 25 20 15 10 5 0 0 1000 2000 3000 4000 [MAO]/[Ti] Fig.4. Influence of MAO/Ti ratio on the catalytic activity of Cat.A/MAO for propylene-co-ethylene polymerization in toluene at 0 oC Fig. 5. 125 MHz 13C-NMR spectra of polymer obtained with [t-BuNSiMe2Flu]TiMe2/MAO at 0 oC Table 1: Results of homo- and copolymerization of propene and ethene with [t-BuNSiMe2Flu]TiMe2/ MAOa Entry Timeb Timec Yield Activity, kg Mnd Mw/Mnd polymer/mol.Ti.h x 10-4 h h g 9e - 1.2 0.24 10.3 f f 10g 1.0 - 0.60 30.0 23.0 1.08 11g - 0.5 0.90 30.0 42.9 1.18 12g - 1.0 0.96 24.0 46.5 1.21 13g - 1.5 1.24 24.8 49.3 1.22 14g - 2.0 1.48 24.7 51.6 1.19 15g - 2.5 1.42 20.3 52.8 1.19 Polymerization conditions: toluene = 100 ml; Ti = 20 mol; MAO = 8.0 mmol; C3H6 concentration = 1.0 mol/l, C2H4 = 1.0 atm; 0 oC. bPolymerization time of propylene homopolymerization. cCopolymerization time after the addition of ethylene. dNumber average molecular weight (Mn) and molecular weight distribution (Mw/Mn) determined by GPC using universal calibration and polystyrene standards. eHomopolymerization of C2H4. f Not determined. gSamples taken at intervals during polymerization. a Table 2: Effect of MAO/Ti ratio on productivity and livingness entry MAO/Ti Activity Mw/Mn Kgpol/mol.Ti.h. 16 500 28.5 1.21 17 1000 35 1.10 18 1500 40.2 1.27 19 2000 38 1.65 20 2500 25 2.10 21 3000 10 2.16 Journal of Scientific & Industrial Research VOLUME 65 578 Living copolymerization of propylene and ethylene with [tBuNSiMe2Flu]TiMe2/MAO catalyst system: Effect of MAO/Ti ratio NUMBER 7JULY 2006 Propylene-co-ethylene polymer (Mn=528,000, Mw/Mn=1.19) was synthesized using [η1: η3-t-BuNSiMe2Flu]TiMe2 (Cat A)/methylaluminoxane (MAO) system at 0°C. Effect of MAO/Ti ratio on catalyst productivity was investigated and living nature of copolymer was maintained until the ratio reached 2000. 13C-NMR and GPC have been used to characterize the copolymer. IPC Code: C08F38/02 E O Dare, G A Olatunji, D S Ogunniyi, S O Oguntoye & J T Bamgbose1