jws-hep.217751
advertisement

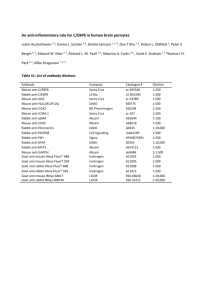
Supplementary Experimental Procedures Materials Chemicals and reagents were from Sigma Chemical Co., St. Louis, MO and Invitrogen Corp., Carlsbad, CA; collagenase type 1 from Worthington Biochemical Corporation, Lakewood, NJ; cell culture medium and supplements from Gibco BRL Life Technologies, Grand Island, NY; hormones from Reprotech Inc., Rocky Hill, NJ or Upstate Biotechnology, Lake Placid, NY; and FBS from Gemini Bio-Products, Woodland, CA. Fixation conditions used for immunohistochemistry Cell suspensions or cultured cells were fixed on cytospins or 2-well chamber slides by incubation in ice-cold 4% paraformaldehyde for 10 min or cold acetone (-20ºC) for 5 min. After fixation in paraformaldehyde, cytospins/chamber slides used for detection of cytoplasmic markers were incubated in 0.1% Triton X-100 for 10 min. Immunohistochemical detection of Thy-1, AFP, CK-19, albumin (Alb), E-cadherin, and CXCR4 Thy-1, AFP, Alb, and CXCR4 detection. After fixation, cytospins were blocked in 2% donkey serum, 1% BSA and 0.05% Tween. Cytospins were stained with mouse anti-Thy-1 (1:10; BD), rabbit anti-AFP (1:100; LabVision), rabbit anti-Alb (1:100; ICN) or rabbit anti-CXCR4 (1:100; Torrey Pines) as primary antibody. Secondary antibody was CyTM3-conjugated donkey antimouse IgG or CyTM3-conjugated donkey anti-rabbit IgG (1:100; Jackson). CK-19 and E-cadherin detection. Cytospins/chamber slides were blocked in 2% goat serum, 1% BSA and 0.05% Tween. Cytospins/chamber slides were stained with mouse anti-CK-19 (1:10; Progene) or mouse anti-E-cadherin (1:50; BD) as primary antibody. Secondary antibody was Biotin-conjugated goat anti-mouse IgG2 (1:50; Nordic), followed by incubation with Streptavidin-CyTM2 (1:100; Rockland). Oertel et al. 2 Simultaneous detection of AFP/Thy-1, Ki-67/Thy-1, Alb/Thy-1, CD45/Thy-1, Alb/CD45, Ki67/AFP, CXCR4/AFP, and CXCR4/Thy-1/AFP AFP/Thy-1, Ki-67/Thy-1 and Alb/Thy-1 double staining. Cytospins/chamber slides were blocked in 2% donkey serum, 1% BSA and 0.05% Tween. After incubation with rabbit anti-AFP (1:100; LabVision), rabbit anti-Ki-67 (1:800; LabVision) or rabbit anti-Alb (1:100; ICN) and mouse anti-Thy-1 (1:10; BD) as primary antibodies, cytospins/chamber slides were stained with CyTM2conjugated donkey anti-rabbit IgG and CyTM3-conjugated donkey anti-mouse IgG (1:100; Jackson) as secondary antibodies. CD45/Thy-1 simultaneous detection. Cytospins/chamber slides were blocked in 2% goat serum, 2% rabbit serum, 1% BSA and 0.05% Tween. Cytospins/chamber slides were stained with mouse anti-CD45 (1:50; Serotec) and mouse anti-Thy-1 (1:10; BD) as primary antibodies. Secondary antibodies included Biotin-conjugated goat anti-mouse IgG2 (1:50; Nordic) and TRITC-conjugated rabbit anti-mouse IgG1 (1:100; Rockland). For CD45 detection, cytospins were stained with Streptavidin-CyTM2 (1:100; Rockland.). Alb/CD45 double staining. After blocking in 2% goat serum, 2% donkey serum, 1% BSA and 0.05% Tween, cytospins were stained with rabbit anti-Alb (1:100; ICN) and mouse anti-CD45 (1:50; Serotec), CyTM3-conjugated donkey anti-rabbit IgG (1:100; Jackson) and Biotinconjugated goat anti-mouse IgG2 (1:50; Nordic) thereafter, followed by incubation with Streptavidin-CyTM2 (1:100; Rockland). Ki-67/AFP and CXCR4/AFP double staining. Cytospins/chamber slides were blocked in 2% donkey serum, 1% BSA and 0.05% Tween. Cytospins/chamber slides were stained with rabbit anti-CXCR4 (1:100; Torrey Pines) or rabbit anti-Ki-67 (1:800; LabVision) and sheep anti-AFP (1:200; Nordic) as primary antibodies. Secondary antibodies included CyTM3-conjugated donkey anti-rabbit IgG and CyTM2-conjugated donkey anti-sheep IgG (1:100; Jackson). Oertel et al. 3 CXCR4/Thy-1/AFP simultaneous detection. After blocking in 2% donkey serum, 1% BSA and 0.05% Tween, cytospins were stained with rabbit anti-CXCR4 (1:100; Torrey Pines), mouse anti-Thy-1 (1:10; BD) and sheep anti-AFP (1:200; Nordic). Secondary antibodies included CyTm2-conjugated donkey anti-rabbit IgG, CyTm3-conjugated donkey anti-mouse IgG, and CyTm5-conjugated donkey anti-sheep IgG (1:100; Jackson). Stromal cell-derived factor 1α (SDF-1α) detection in liver tissue Five m sections of formalin-fixed/paraffin-embedded liver tissue were deparaffinized in xylene and rehydrated by decreasing ethanol concentrations. Sections were treated by microwave antigen retrieval for 15 min in 10 mM citrate buffer, pH 6. After blocking in 2% donkey serum, 1% BSA and 0.05% Tween, sections were stained with mouse anti-SDF-1α (1:50; R&D) as primary antibody. Secondary antibody was CyTm3-conjugated donkey anti-mouse IgG (1:100; Jackson). Reverse Transcription-Polymerase Chain Reaction (RT-PCR) and Western blot analysis RT-PCR analysis. Total RNA was extracted from fetal liver suspensions using Trizol reagent, following the manufacturer’s protocol. RNA was reverse transcribed using SuperScriptTM III reverse transcriptase and oligo-dt primers to synthesize cDNA (Invitrogen Corp.). Primers used for these analyses are shown in Supplementary Table 1. cDNA was amplified by Ampli-Taq Gold DNA polymerase (Applied Biosystems, Foster City, CA) for 10 min at 95˚C, followed by 20-40 cycles at 94˚C for 30 sec, 55˚C for 60 sec, 72˚C for 60 sec and a final cycle of 72˚C for 7 min (see Supplementary Table 1). Western blot analysis for detection of AFP and CK-19. The proteins were resolved by 10% reduced SDS-PAGE and transferred to nitrocellulose membranes using a Semi-Dry Transfer Cell system (Bio-Rad Laboratories Inc., Hercules, CA). After blocking in 5% non-fat dry milk, the membranes were incubated with rabbit anti-AFP (1:1000; LabVision) or mouse anti-CK-19 Oertel et al. 4 (1:500; Progene). Secondary antibody was horseradish peroxidase-conjugated donkey anti-rabbit IgG or sheep anti-mouse IgG (1:5000; Amersham Biosciences UK Limited, Little Chalfont Buckinghamshire, UK). Horseradish peroxidase was detected using SuperSignal® West Femto Maximum Sensitivity Substrate (Pierce Biotechnology Inc., Rockford, IL). Determination of marker expression after immunohistochemistry To calculate the percentage of cells expressing each marker in the Thy-1+ and Thy-1- cell fractions, 1084 ± 72 immunohistochemical stained cells in 35 ± 3 separate microscopic fields were counted for each analysis. Data were analyzed using SigmaStat 2.01 software (SPSS Scientific, Erkrath, Germany) and are reported as the mean ± SEM.