Kidneys, Ureters, and Suprarenal Glands
advertisement

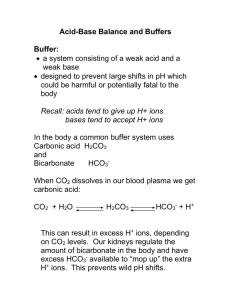
Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 1 of 7 Renal Regulation of Acid-Base Balance I. Session 8 Overview Dr. Tune presented the first part of acid-base regulation in the respiratory system. Ventilation is adjusted to control the CO2 in the blood, thus adjusting the extracellular pH. The acid-base lectures for the kidneys present their role. If the kidneys don’t excrete acid, metabolic acidosis results due to decreased arterial pH. This acidosis is caused by fixed acids, which are generated by metabolism. Definitions: acids, bases, pH Chemical buffer systems that minimize the change in pH in extracellular fluid and inside the cells themselves. Metabolic sources of acid: ‘volatile’ acids (in equilibrium with CO2) vs . ‘fixed’ acids—those that are excreted by the kidney and cannot be converted to CO2. Mechanisms to maintain pH of body fluids Bicarbonate buffer system: the most important buffer; an open buffer system Renal tubular H+ secretion: proximal tubule—critical for the reabsorption of bicarbonate, collecting duct Reabsorption of filtered bicarbonate Excretion of H+ as titratable acid, ammonium II. Normal arterial acid-base values Remember that pH = -log [H+] Normal ranges will be provided for the cases on the exam. If P CO2 is outside its normal range, then there’s a respiratory problem or respiratory compensation for a metabolic problem. Mean Range* pH 7.40 7.35-7.45 [H+], nmol/L 40 45-35 PCO2, mmHg 40 35-45 [HCO3 ], mEq/L or mM/L 24 22-26 *The range extends from 2SD below to 2SD above the mean and encompasses 95% of the population of healthy people. III. Importance of maintaining pH Maintenance of hydrogen ion concentration. H+: reactant or product of many enzymatic reactions (i.e. dehydrogenase reactions); affects reaction rate. Remember that H+ is a cation. It binds to an anionic charge. If this charge is within a protein, the binding can affect the protein’s shape and function. As you move away from the optimum pH, enzyme’s activity declines. Effects of H+ on electrostatic charge of proteins: affects protein function Effects of H+ on free plasma concentrations of other cations (e.g. Ca2+) Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 2 of 7 Effects of H+ on intracellular cation concentrations (Na+/H+, K+/H+ exchanges). If the hydrogen ion concentration is increased in extracellular fluid, the hydrogen ions enter the cell. So, another ion will leave the cell to maintain equilibrium. This ion is K+ because it’s the most abundant in the cell. IV. Review of acid-base chemistry A. Acids Lewis acids are chemicals that release H+: HA ↔ H+ + A- The acid and its conjugate base are in equilibrium. Dissociate to some extent in aqueous solution. Strong acids dissociate more than weak acids. Strong acids (i.e. HCl) dissociate completely providing a high [H+] and a low pH. Organic acids are weaker, so they dissociate less resulting in a relatively higher pH. Acid strength is expressed by its association constant, Ka: [H+] [A-] Ka = [HA] Strong acids have a high Ka while weak acids have a low Ka. Two kinds of acid in body: ‘volatile’ and ‘fixed’ 1. Volatile acid: – The only one is the major acid in the body, carbonic acid: H2CO3. This can be removed by respiration. – In chemical equilibrium with CO2, a volatile gas: H2CO3 ↔ CO2 + H2 O – Pulmonary ventilation controls H2CO3 concentration in body fluids by removing CO2. 2. Fixed acids: non-carbonic acids generated metabolically (e.g. sulfuric, phosphoric acids) – Initially neutralized by buffers in body fluids Ultimately excreted in urine – Cannot be ventilated away by the lungs because they are not in – equilibrium with CO2. Metabolic sources of H+ – Oxidative metabolism is the major source of H+: CO2 (c. 15,000 mEq/day) CO2 + H2O ↔ H2CO3 ↔ H+ + HCO3– Nonvolatile (fixed) acids: 40-80 mEq/day – Glycolysis: lactic acid (pKa 3.9). Lactic acid readily dissociates due to its low pKa, so it yields plenty of H+ and lowers the pH. – Incomplete oxidation of fatty acids: ketone acids (pKa c. 4.5) – Protein, nucleic acid, phospholipid metabolism generates sulfuric, phosphoric, hydrochloric acids. These are strong acids and almost completely dissociate in the body fluids. Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 3 of 7 – Fixed acids cannot be removed from body by ventilation; therefore, they must be eliminated by the kidneys (urinary excretion). – How is CO2 a source of acid? It readily combines with H20 to form carbonic acid. This dissociates as already seen. B. Bases: Are proton acceptors. Chemicals that combine with (accept) H+: B + H+ → BH + C. The Henderson-Hasselbalch equation relates the -log10 of [H+], i.e. pH, to: – the -log10 of the association constant, i.e. pKa, and – The ratio of concentrations of the conjugate base and weak acid. – pH = pKa + log [A-] / [HA] D. Chemical buffer systems Mixture of weak acid and its conjugate base in aqueous solution Important because they minimize the changes in pH. Chemical buffers minimize but don’t completely prevent pH changes caused by strong acid or base Respiration and the kidney function help correct this pH change. Ability (‘strength’) of buffer to minimize pH changes depends on: – Concentrations of buffer system components – Nearness of buffer’s pKa to pH of solution—the most effective buffers have a pKa equal to or almost equal to the solution’s pH. Example: (see ppts for plot) phosphate buffer system H2PO4- (monobasic phosphate) ↔ HPO42- (dibasic phosphate) + H+ This is important in extracellular fluid. Its pKa = 6.8. This means that at a pH = 6.8, [H2PO4-] = [HPO42-]. If a strong acid is added, there will only be small changes in the pH initially. Once increasingly more acid is added, there’s a larger decrease in pH as seen by the steeper slope of the titration curve. If a strong base is added, again there are gradual changes in the pH initially. Then, there are greater changes when increasingly more base is added. The phosphate buffer system is effective because its pKa, 6.8, is close to the arterial pH = 7.4, close to the buffer pKa. V. 3 lines of defense against pH changes A. Respiration—ventilates CO2. This removes H+ due to equilibrium: CO2 + H2O ↔ H+ + HCO3B. Kidneys—respond slowly. They excrete excess acid or base in the urine to completely restore pH to normal. This takes hours or days. C. Chemical buffers Are the first line of defense in intra and extracellular fluid by combining with H+ produced by metabolism. Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 4 of 7 See ppts for the table of the reactions. Extracellular Fluid: Bicarbonate/CO2 is the major buffer. Inorganic phosphate is limited because the phosphate concentration in extracellular fluid is far less than the bicarbonate concentration. Intracellular Fluid: Organic phosphates like ATP and phosphocreatine are used. Bicarbonate/CO2 is a little less effective here because there’s less bicarb in the intracellular fluid. Bicarbonate system is the major EC buffer – It’s easily monitored clinically. – Equilibrium between H2CO3 and HCO3- (pKa = 3.5): Plugging into the Henderson Hasselbach equation gives [HCO3-] pH = 3.5 + log [H2CO3] – H2CO3 concentration is very low and it is in equilibrium with CO2 and H2O. CO2 concentration is 400*[H2CO3], thus [HCO3-] pH = 3.5 + log [CO2]/400 , or [HCO3-] [CO2] pH = 6.1 + log Know this form of the equation! It’s important clinically. – CO2 concentration is related to PCO2. For each mm Hg PCO2, 0.03 millimolar CO2 is in solution at 37°C. Thus: [HCO3-] pH = 6.1 + log 0.03 * PCO2 – The advantage: [HCO3-] and PCO2 are easily measured. Why is bicarbonate buffer system so powerful? 1. Components (HCO3-, CO2) are abundant. [HCO3-] = 22 – 26 mEq/L. It’s the 2nd most abundant anion in the Extracellular fluid. Metabolism produces lots of CO2. 2. Bicarbonate buffer system is ‘open’; concentrations of HCO3- and CO2 are readily adjusted by respiration and renal function: oxidative metabolism kidneys CO2 ↔ HCO3ventilation kidneys Bicarbonate pKa = 6.1. This is fairly far removed from the arterial pH = 7.4. However, the bicarbonate buffer system is still powerful because it is open. Oxidative metabolism produces CO2 which can be removed by the lungs. Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 5 of 7 The kidney can excrete bicarbonate (remove excess base) to lower the pH. Reabsorption of bicarb raises the pH. Response of bicarbonate system to strong acid—see ppt for bar graph. You don’t have to memorize the numbers on the plot. It is an illustration of an open buffer system. The height of the columns corresponds to the concentration of CO2 and HCO3-. You have a normal subject with [HCO3-] = 24mEq/L. Add 10mM of HCl. If there’s no buffering, the pH is lowered to 2. Because there’s buffering, it’s decreased to 6.2. The bicarbonate concentration is lowered by 10 and the CO2 is increased by 10. If the buffer system is a closed system, it stops here and the person dies. Since it’s an open system, the lungs help out. The person begins to hyperventilate to get rid of CO2. The pH now increases to 7.17. Lowered pH means a higher hydrogen ion concentration. These ions themselves stimulate peripheral chemoreceptors and further increase ventilation. This brings the pH to about 7.29, which is close to normal but still shows excess acid. The kidneys excrete the excess acid and make bicarb to replace that which was used to neutralize the acid. The pH and [bicarb] are returned to normal. VI. Renal regulation of pH via urine Urine pH range: 4.5 ( in response to acid load) - 8.0 (in response to excess base) A. Renal response to excess acid: All of filtered HCO3- is reabsorbed Additional H+ is secreted into lumen, excreted in 2 forms: 1. titratable acid, 2. ammonium B. Renal response to excess base: Too much base means too much bicarbonate Incomplete reabsorption of filtered HCO3- Not all can be reabsorbed, so some spills into urine. Decreased H+ secretion Secretion of HCO3- in collecting duct by β-intercalated cells Each day, 40-80 mEq H+ are excreted in urine If urine pH = 4.5 and 2 L urine are excreted per day, how much H+ is excreted as free H+? At ph 4.5, [H+] = 30uM, so amount is 30uM * 2L = 60 uM. Only a small amount is released. If this free H+ was the only way to remove fixed acid, you’d have to eliminate 2000L/day. Most H+ is excreted in combination with urinary buffers. Two types: 1. Titratable acid: conjugate bases of metabolic acids (phosphate, sulfate) accept H+ in lumen. The acids are filtered into the tubular lumen and combine with H+. Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 6 of 7 2. Ammonia, generated by tubular epithelium during metabolism. It combines with H+ to form ammonium. Charged compounds are trapped in the lumen. C. Total renal H+ excretion H+ excretion = urinary excretion of titratable acid + ammonium - HCO3 Typical rates (mEq/day): 24 + 48 - 2 = 70 mEq/day Note: HCO3- excretion is equivalent to adding acid to body fluids because the base is being lost from the body. Again, don’t memorize the values. They depend on how much acid the body produces. If the kidney can’t reabsorb bicarbonate, acidemia results from the loss of base. D. Acidification of urine begins in proximal tubule (figure) (Berne & Levy Fig 44-2, p. 766) A Na+/H+ counter transport is used. As Na+ is reabsorbed, H+ is excreted. The Na+/K+ pump is the energy source for this because it establishes the Na+ concentration gradient. There is also a H+--ATPase pump to pump out the H+. E. Luminal pH along nephron (figure) The proximal tubule secretes lots of H+, but there is only a slight change in pH. This is because the tube is leaky and a large concentration gradient of H+ cannot be maintained. There is no further change in pH until the fluid reaches the collecting duct. There H+--ATPase pumps expel H+. Summary: proximal tube secretes lots more H+ than the collecting duct. Its pH has a small change (pH 6.7) because 1. it’s leaky and 2. most of the secreted H+ is used to reabsorb bicarbonate. The collecting duct has a tighter epithelium and is not leaky, so the pH drops to 4.5 there. VII. Reabsorption of HCO3A. Collecting ducts can secrete H+ or HCO3- (See B&L Fig 44-3, p. 767) α-intercalated cells actively secrete H+: H+ ATPase—ATP hydrolysis directly provides energy. Up to 900 fold [H+] gradient—tight junctions help this. β-intercalated cells actively secrete HCO3- to eliminate excess base if body is alkaline. B. Tubular reabsorption of filtered HCO3 If this fails, it effectively adds H+. This is only ok if the body is alkalotic. At 25 mEq/l plasma concentration, c. 4500 mEq of HCO3- are filtered into nephrons per day, if GFR = 180 L/day. Excretion of HCO3- has same effects as gaining H+; excretion of even small fraction of filtered HCO3- would acidify body fluids Normal individuals: all of filtered HCO3- must be recaptured Renal #20 Thurs, 03/20/03, 8am Dr. Mallet Jennifer Uxer for Dale G. Page 7 of 7 If arterial pH is too high, kidneys respond by incompletely reabsorbing HCO3-, and it spills into the urine. C. Mechanism to reabsorb filtered HCO3 (See B&L Fig 44-2, p. 766) Most of the H+ (~90%) secreted is used to reabsorb the bicarbonate in the proximal tubule. The rest of the bicarb is reclaimed in the loop of Henle. How does filtered bicarbonate cross the membrane? 1. H+ is secreted mainly by Na+/H+ exchange 2. Combines with HCO33. Cell membrane has carbonic anhydrase which causes the dissociation of carbonic acid into CO2 and H2O. 4. CO2 then crosses the cell membrane. 5. Carbonic anhydrase within the cell then aids in reforming the carbonic acid on the other side of the membrane. 6. Carbonic acid dissociates. Bicarbonate is reclaimed and CO2 is expelled. D. Important features of HCO3- reabsorption Net effect: HCO3- reabsorption. HCO3- is temporarily converted to CO2 Ultimately dependent on Na+,K+ ATPase—provides the energy for the Na+/H+ exchange. Process does not result in net secretion of H+ —regulated when CO2 comes in. By this mechanism, 90% of filtered HCO3- is reabsorbed in proximal tubule, most of remainder in thick ascending limb A saturable process: at [HCO3-] > 26 mEq/l, some is excreted in urine E. Saturation of HCO3- reabsorption (graph) This shows that at [HCO3-] > 26 mEq/l the reabsorption process saturates.