Evaluation of Norepinephrine Effects on Phosphorous
advertisement

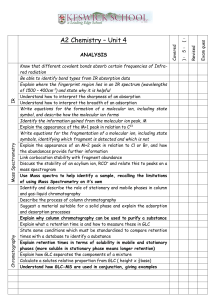
Evaluation of Norepinephrine Effects on Phosphorous Metabolism with 31P NMR Erin Haase, and Leah Tucker 12/14/04 Abstract Norepinephrine (NE) is an adrenergic drug that increases cellular demands for ATP utilization and synthesis. This increases the need for phosphagen breakdown. 31P NMR was used to measure whether NE (100mM) increased earthworm phospholombricine utilization. Spectra from the 31P NMR showed a significant increase in the free phosphate (p=0.0005) signal at ppm 2-1, and a significant decrease in the signal intensity for the terminal phosphate of phospholombricine (PL) at ppm 4-6 (p=0.0004). This study suggests that phospholombricine NE is an earthworm stimulant and that 31P NMR can be used to evaluate changes in earthworm phosphagen status. Introduction Norepinephrine is an adrenergic drug that evokes a physiological response similar to those produced by the sympathetic adrenergic nerves. (Levy 1965) There are different types of adrenergic receptors that are affected by norepinephrine. (Bennett 1996) The two main types, αadrenergic and β-adrenergic receptors have different physiological effects on the organism. (Bennett 1996) Alpha-adrenergic receptors are mainly excitatory. Humans use the phosphagen phosphocreatine as an inorganic phosphorous donor to maintain the ATP supply. (Figure 1) Phosphagens are energy rich phosphate compounds closely linked to ATP. (Fox 1984) Phosphagens are stored in muscle cells and when they are dephosphorylated, a large amount of energy is released (7.3kJ/mole). (Brown 1992) This energy released is used to re-phosphorylate ADP to ATP. Once the energy supplies of phosphocreatine run out the body turns to glycolysis and aerobic energy sources. Figure 1 Mechanism of action for the donation of inorganic phosphorous from phosphocreatine to ADP which forms ATP Humans use phosphocreatine; earthworms use a different phosphagen, as their inorganic phosphorous source, for maintaining ATP supplies, called phospholombricine. (Watts 1968, Hoffmann 1981) Phospholombricine has a terminal phosphate (PL) group similar to that of phosphocreatine which is readily cleaved off when energy and a Pi are required to regenerate ATP from ADP in the earthworm. (Figure 2) ADP then accepts the high energy phosphate group to regenerate ATP from ADP. (Suzuki 1994) ATP is a high energy source when its γ phosphate is cleaved there is a large amount of energy released, 7.3 kJ, which is used to attach inorganic phosphorous to ADP. (Nelson 2005) This Phosphate is indicated as (PL) Phosphate indicated as (Pi) Figure 2 the mechanism of action for the phospholombricine (PL) peak attaching to adenosine diphosphate to form adenosine tri-phosphate 31 P NMR can be used to evaluate changes in the Pi peak (2-1ppm), the terminal PL of lombricine (4-6ppm), and the internal structural phosphate (L-PL) (0ppm). (Figure 2) Thus, in 31P-NMR spectra two peaks are detected, the phosphoryl group of the phosphagen and the structural phosphodiester of lombricine and phospholombricine. (Wilson 1992) Earthworms should be an excellent organism for studying the metabolic changes following exposure of norepinephrine. Adrenergics, like norepinephrine and epinephrine are ubiquitous to many organisms including earthworms. (Bieger 1972) Phospholombricine, unique to earthworms, has a structural phosphate that can be measured as an internal marker with 31P NMR. (Wilson 1992) Earthworms also have α adnrenergic receptors which are necessary for norepinephrine to have an effect on earthworm metabolism. (Tanaka 1983) Earthworms were selected for this experiment because they are invertebrates, meaning permission to do the study from IACUC is not needed, earthworms are also readily available. This study used 31P NMR to determine if NE has a stimulatory effect on earthworm phosphorous metabolism. Stimulation should cause increased ATP usage. Norepinephrine exposure should also cause the terminal phosphate on phospholombricine to be cleaved off and donated to ADP in order to maintain ATP levels. It should be possible to measure changes in the terminal phosphate relative to the internal phosphorous that phospholombricine contains. Materials and Methods Animals Specimens of the earthworm, Eisenia fetida were obtained from the West End bait shop in Winona, MN. Earthworms were placed in an ice bucket while in the plastic container they were purchased in until use for 31P-NMR. Earthworms were purchased on the same day 31P-NMR experiments were performed. 31 P-NMR Spectroscopy The settings for the experiment were based on the settings used by Wilson, et. al., 1992. 31 P-NMR spectra were collected using a JEOL ECX-300 spectrophotometer with variable temperature capability operating at 121.65 MHz. Each scan represented 2,000 scans and 512 data points. There was proton decoupling and the sweep width was 8.22368 kHz. The temperature was set at 8° C. Prior to adding any earthworms, the NMR was gradient shimmed on 2 ml of 50% D2O and 50% H2O. Then 2 earthworms were added to each sample to be run. The earthworms were placed in a 5 ml tube immediately prior to the experiment, and the periods of data collection were not longer than 20 minutes. The NMR was set up as follows: The LF course was set to X, the LF Tune was set to 2294, and the probe-match was set to 3448. On the computer desk top click on the icon shortcut to delta. Then click on the magnet picture which is located to the left of the ? icon. Click on sample then select the solvent which for this experiment was D2O. Place a 5 mm NMR tube which has a 50% D2O and 50% H2O solution into the NMR and gradient shim. The button to do this is located under the lock control section and has a picture of a padlock which is locked with some green lines in the background. To load the sample press the ↓ green arrow. After the sample is loaded press the auto lock button under the lock control section. Then press experiment which is located in the spectrometer control box. Under filename, select the single pulse.ex2. Select the auto_gain box. Under Instrument select the solvent used which was D2O. Under Acquisition select Phorphorous31 in the x_domain, x_offset is 0, x_sweep is 54ppm, x_points is 512, scans is 2000, prescans is 4, and mod_return is 1. Under the section Pulse, the x_angle is 78°, and the relaxation delay is set to 0.5s. After this is set press save so you can retrieve your settings for future experiments instead of resetting everything. Select the Submit button. Set up the external cooling by attaching the liquid nitrogen carboy to the NMR machine. Then under Sample set the Temperature to 8°C. Under the Spectrometer Control box hit the GO button which is surrounded by a green circle. While the set-up of 31P NMR was being completed, norepinephrine was solubilized in double de-ionized water, making a 0.1M solution. Norepinephrine was purchased from Sigma Chemical Co. A 10 cm petri dish with a 9 cm piece of Whatmann’s filter paper in it, and 5 ml of 0.1M norepinephrine(sample), or water(control) distributed onto the filter paper was set up for each sample. Two earthworms were placed on each petri dish, and 5 ml of 0.1M norepinephrine was distributed onto the filter paper. The control was set up in the same manner except there wasn’t any norepinephrine added to the double deionized water. The earthworms were placed on the papers for five minutes each. After exposure to norepinephrine, or water, the worms were placed as gently as possible into a 5 ml NMR tube. If a 10 ml tube can be used it would be beneficial as an alternative to the 5 ml tube. This will prevent an undesired amount of stress on the earthworm from the insertion into the tube. This stress may cause an unwanted increase in ATP synthesis. Statistical Data The mean and standard deviation of 31P peak identity, ppm, and intensity was taken from the 31P NMR spectra. The intensity of the internal phosphate of the phospholombricine (L-PL) peak was used as 100% because it is found in every molecule of phospholombricine in the reaction regardless of whether the terminal Pi is attached (LPL) or not (LP). The percent of the PL and Pi peaks were taken as percentages of the LPL. The percentage that the Pi varied between the control and NE treated worms was placed into Jump and statistical analysis was performed. The percentage that the PL peak changed between control and NE treated worms was also placed into Jump and addition statistical analysis is performed. Results 31 P NMR spectra for norepinephrine treated and control earthworms, are represented in figures 3 and 4. The ppm ranges found in both control and NE spectra for the peaks Pi, PL, L-PL, -phosphate, -phosphate, and -phosphate are displayed in table I. Statistical data gathered from the 31PNMR spectra is shown in table 2. Table 2 also shows percent comparisons found between the phospholombricine (PL) peaks and the inorganic phosphorous (Pi) peaks as a percent of the internal phosphate (L-PL). The graph created in Jump expressing the control and norepinephrine data for the Pi is found in figure 6. The graph created in Jump expressing the control and norepinephrine date for PL is found in figure 7. Statistical analysis results found by Jump for the Pi data are shown in table III, and those for PL are shown in table IV. Control 31P NMR Trials Figure 3 Control 1, 2, and 3 represent the 31P NMRs. The peak indicated with orange is the internal phosphorus found in phospholombricine. This peak for statistical purposes was 100%. The peak indicated with the pink is the terminal PL peak. This is the phosphorus at the end of the phospholombricine while still attached. The peak indicated in green is Pi. This is the phosphorus while not attached to the phospholobricine. Norepinephrine 31P NMR Trial Data Figure 4 These are the three norepinephrine 31P NMRs. The orange peak is the internal phosphorus of the phospholombricine. The green peak is the Pi, which is the free phosphorus. The pink peak is the terminal PL peak. This is the peak that indicates the second phosphorus molecule of the phospholobricine. It indicates the phosphorus is still attached to the phospholombricine. peak assignments Pi PL L-PL -phosphate -phosphate -phosphate ppm range 2 to 1 ppm -4 to -6 ppm 0 ppm -6 to -8 ppm -9 to -12 ppm -17 to -20 ppm Table I ppm values for the Pi (free phosphate) peak, PL (phospholombricine) peak, L-PL (internal phospholombricine) peak, and the adenosine phosphate peaks found in the 31P NMR Peaks Trial One Represented % Control L-PL 100% PL 77.7% Pi 22.2% Trial Two % Control 100% 75.9% 24.1% Trial Three% Control 100% 73.3% 26.6% Trial Four % NE 100% 52.0% 48.0% Trial Five % NE 100% 56.3% 43.7% Trial Six % NE 100% 50.6% 49.6% Table II Percent comparisons found between the PL and Pi 31P NMR peaks (figures 4 and 5) in the tested worms, L-PL is representative of 100%, the PL and Pi are percentages of the L-PL peak % inorganic Phosphate 50 45 40 35 30 25 20 Control Treated Column 1 Figure 5 Control and NE treated differences in the % inorganic phosphate (Pi) peaks found on the 31 P NMR spectra 80 % Phospholobricine 75 70 65 60 55 50 Control Treated Column 1 Figure 6 Control and NE treated percentage differences for the phospholombricine (PL) peak found on the 31 P NMR spectra t-Test Results for Inorganic Phosphate (Pi) Standard Error Differential Upper Curve Differential Lower Curve Differential Confidence Probability (p value) 2.174 28.838 16.764 0.95 = 95% 0.0005 Table III t-test results given by Jump, the standard error differential gives the range of error probable for each % difference of Pi peaks between the NE treated and the control spectra, upper curve differential gives the upper end of the probable curve for the difference in Pi peaks, lower curve differential gives the lower end of the probable curve for the Pi peaks, the confidence gives level of confidence that future data collected will be between the upper curve and lower curve differential t-Test Results for Phospholombricine (PL) Standard Error Differential Upper Curve Differential Lower Curve Differential Confidence Probability (p value) 2.138 -16.73 -28.604 .95 = 95% .0004 Table IV t-test results given by Jump, the standard error differential gives the range of error probable for each % difference of PL peaks between the NE treated and the control spectra, upper curve differential gives the upper end of the probable curve for the difference in PL peaks, lower curve differential gives the lower end of the probable curve for the Pi peaks, the confidence gives level of confidence that future data collected will be between the upper curve and lower curve differential Discussion This study suggests that exposure of norepinephrine to organisms with α adrenergic receptors will increase ATP synthesis demand. There was a marked decrease in signal intensity of the PL peak, and the Pi peak following NE exposure rose. (Table II) The PL phosphate of phospholombricine was cleaved and used as a free inorganic phosphate to combine with ADP in the synthesis of ATP. Statistical analysis shows that norepinephrine creates a significant increase in the Pi peak (p=0.0005) and a marked decrease in the PL peak (p=0.0004). This suggests the hypothesis that NE would increase ATP usage resulting in an increased depletion of PL. At the same time, free Pi should accumulate and the signal intensity for this peak increased following NE exposure. In summary, this study showed that the adrenergic drug, NE had an effect on phosphorous metabolism in earthworms. The experiment also showed that 31P NMR can be used to quantitatively measure those changes in phosphorous metabolism of living earthworms that adrenergic drugs cause. This study may be useful in the future to study other adrenergic drug effects, and the methods may also prove beneficial to future 31P NMR uses. Acknowledgements The authors thank Dr. Ted E.F. Wilson for his guidance and suggestions. Special thanks also go to Dr. Thomas W. Nalli for his knowledge and suggestions with NMR procedures, and to Dr. Christopher Malone for his assistance with statistical data. This paper is for fulfillment of the Capstone research project required for graduation from Winona State University. The work done for this paper was performed at Winona State University and supplies were received from the Departments of Biology and Chemistry at Winona State University. Drs. Ted E.F. Wilson and Thomas W. Nalli also supplied required materials. References 1. Levy B., Ahlquist R., Adrenergic Drugs. Drills Pharmacology in Medicine 3rd ed., New York, 1965, pg. 463-480. 2. TenBrook J.A. Jr., Wolf M.P., et. al., Should beta-blockers be given to patients with heart disease and peanut-induced anaphylaxis? A decision analysis. J. Allergy Clin Immunol., 2004 May, 113(5), 977-82. 3. Cammarata G., Weil M.H., et. al., Beta-adrenergic blockade during cardiopulmonary resuscitation improves survival, Crit. Care Med., 2004 Sep, 32(9 Suppl), 440-3. 4. Bennett, Plum, Cecil Textbook of Medicine, 20th ed. Vol. 2, W.B. Saunders Co., Philadelphia 5Wilson E.F., Brown G.G., Drewes C.D., Characterization of phosphorous metabolism during six stages of development in the earthworm Eisenia foetida using 31 P-NMR. Comp. Biochem. Physiol. Vol. 102B, No. 2, pp383-388, 1992. 6. Hoffmann K.H., Inhibition of Tubifex Pyruvate Kinase by storage phosphagens and adenosine triphosphate, Comp. Biochem. Physiol., Vol 70B, 1981, 77-83. 7. Fox E., Sports Physiology 2nd ed., Saunders College Publishing Columbus Ohio, 1984, pgs 14-15. 8. Brown G.G., Drewes C.D., 31P-NMR Analysis of phospholombricine and other phosphorus-containing metabolites in selected freshwater and terrestrial oligochaetes, Comp. Biochem. Physiol., Vol. 102B, No. 2, 1992, 389-396 9. MacLaren D., Creatine, Drugs in Sport 3rd ed., London, 2003. 10. Watts D.C., Variation in enzyme structure and function: the guidelines of evolution. Advan. Comp. Physiol. Biochem., 3, 1968, 1-115. 11. Suzuki T., Furukohri T., Primary Structure of Glycocyamine Kinase and Arginine Kinase From Invertebrates, J. Mol. Biol.,1994, 237, 353-357. 12. Nelson D.L., Cox M.M., Lehinger Principles of Biochemistry 4th ed., 2005, New York, W.H. Freeman and Company. 13. Tanaka K.R., Webb R.A., Octapamine action on the spontaneous contractions of the isolated nerve cord of Lumbricus terrestris, Comp. Biochem. And Phys. Part C: Comparative Pharmacology, 1983, 113-120. 14. Bieger D., Hornykiewicz O., Dopamine in the earthworm Lumbricus terrestris: Enhancement of rhythmic contractile activity, Neuropharmacology, Vol. 11, Iss. 5, Sept. 1972, 745-748.